Meningococcal disease
The meningococcal group focuses on ensuring the safety and quality of a number of licensed meningococcal vaccines and producing standard reference reagents for epidemiology, research and commercial laboratories.
The group also carries out vaccine-based research which includes:
- developing and refining assays for quality control of licensed products
- assessing novel vaccines at a pre-clinical stage
- exploring the surface structure of the meningococcus to better understand the biological mechanisms which influence vaccine effectiveness
Our group is associated with many of the current meningococcal vaccine developments and provides expert advice to key stakeholders including product manufacturers, WHO and other regulatory bodies.
Standardisation
NIBSC provides a range of meningococcal reference materials designed for:
- evaluating post-vaccination sera
- typing of meningococcal isolates by epidemiology laboratories
- quantitative standards for vaccine manufacturers and National Control Laboratories to determine polysaccharide content of vaccines
We evaluate reference material both in-house and through collaborative studies. NIBSC is keen to expand collaborations and develop new standards for the meningococcal community. Novel projects in consideration include nucleic acid typing standards for molecular typing laboratories, and reagents to support the implementation of Bexsero for both evaluation of vaccine serum and for quality control purposes.
Meningococcal vaccines
Neisseria meningitidis is a major cause of meningitis and septicaemia, especially in infants and young adults. Six serogroups (A, B, C, W, Y and X) account for most of the disease, and capsular polysaccharide vaccines offer protection against serogroups A, C, W and Y. A similar vaccine for group X is under development.
Serogroup B disease is the major cause of meningococcal disease in developed countries, however the serogroup B polysaccharide is poorly immunogenic, preventing a similar vaccine being developed for this group. In 2012 a novel vaccine, Bexsero (see below), was licensed for the prevention of group B disease and will soon be used in immunisation programmes.
Conjugate vaccines
Polysaccharide conjugate vaccines have superior immunogenicity to the plain polysaccharide vaccines and have an established safety and efficacy record, enabling physicochemical tests to be used to measure consistency and potency.
Tests carried out at NIBSC ensure:
Bexsero
Bexsero is a novel vaccine, licensed in 2012 for the prevention of group B disease and will soon be used in immunisation programmes.
Bexsero is made up of three recombinant proteins expressed in E.coli and an outer membrane vesicle (OMV) derived from a meningococcal strain. OMVs naturally bleb from the surface of the meningococcus and contain phospholipids, outer membrane proteins and lipopoligosaccharide (endotoxin). The tests carried out at NIBSC ensure:
- safety, confirmed using the monocyte activation test
- potency,using an in vivo assay to demonstrate a batch is non-inferior to a defined reference batch
- consistency, through measurement of LPS content, quantification of key OMV antigens and purity of recombinant antigens
Proteomics
The group has been involved in a number of proteomic projects involving the elucidation of the protein content of OMVs, analysis of the immune murine and human response to OMVs and identification of novel potential vaccine candidates.
We have used various techniques – including one- and two-dimensional SDS-PAGE, MALDI-TOF and tandem mass spectrometry and immunoblotting – alongside the NIBSC proteomics group and external collaborators from the Norwegian Institute of Public Health (NIPH), Professor Christoph Tang and Dr Andrew Gorringe.
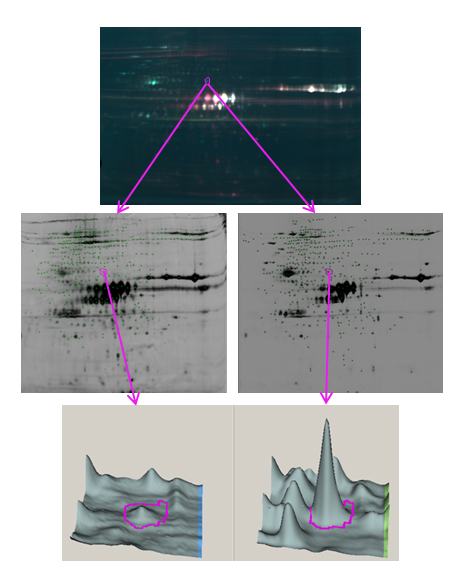
Overview of comparative meningococcal proteomics project using gel based Decyder™ technology
Manipulation of OMV formation for vaccine production
Understanding the biology of vesicle formation in the meningococcus is important in optimising the production and safety of OMV vaccines. The group has studied the mechanisms involved in vesicle formulation, in particular the function of reduction modifiable protein (RmpM), mutation of which leads to a blebbing phenotype.
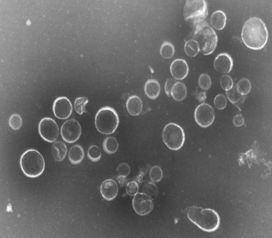
Electron micrograph of native OMVs produced at NIBSC
Collaborators on these projects are Dr Sandra Sanchez and Dr Jeremy Derrick.
Novel vaccine candidates
The group has been involved in a number of collaborations investigating various approaches.
MenPF: an OMV vaccine based on PorA and FetA that has been used in a Phase I clinical trial.
This work was funded by a Strategic Translation Award 082102/Z/07/A by the Wellcome Trust to Prof Andrew Pollard, Prof Martin Maiden, Prof Jeremy Derrick and Prof Ian Feavers.
Viral vectors: Early studies of pre-clinical immunogenicity with PorA, FetA and fHbp in virus-like particles and an adenovirus vector.
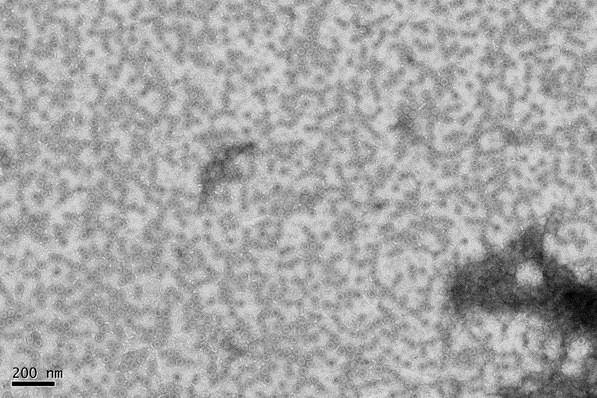
Electron micrograph of VLPs courtesy J Derrick
Iron uptake proteins:This project investigated different prerequisites that are key to supporting the case for haemoglobin receptors as potential vaccine candidates.
This work was funded by the Meningitis Research Foundation to Dr Chris Bayliss.