NIBSC merges with the MHRA
1 April 2013
The National Institute for Biological Standards and Control (NIBSC), previously part of the Health Protection Agency (HPA), is now a new centre of the Medicines and Healthcare Products Regulatory Agency alongside the Clinical Practice Research Datalink (CPRD).
The MHRA and NIBSC have worked closely together for many years and have common interests in managing risks associated with biological medicines, facilitating development of new medicines safely and effectively, and maintaining UK expertise and ability to contribute to assuring the quality and safety of medicines in Europe and beyond.
These developments have created a new organisation that is a world leader in supporting science and research and the regulation of medicines and medical devices, strengthening the support provided to the UK’s medicine’s industry.
The Medicines and Healthcare Products Regulatory Agency family
The new Medicines and Healthcare Products Regulatory Agency group consists of:
- MHRA Regulatory, who protect health and improve lives by ensuring that medicines and medical devices work, and are acceptably safe; focusing on the core activities of product licensing, inspection and enforcement, and pharmacovigilance
- The Clinical Practice Research Datalink (CPRD), which gives access to an unparalleled resource for conducting observational research and improving the efficiency of interventional research, across all areas of health, medicines and devices. CPRD joined the MHRA in 2012.
- The National Institute for Biological Standards and Control, world leaders in assuring the quality of biological medicines through product testing, developing standards and reference materials and carrying out applied research.
The role and remit of each of the three organisations
MHRA
The MHRA is responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe. Underpinning all our work lies robust and fact-based judgements to ensure that the benefits justify any risks. This is achieved through:
- authorising medicines before they can be marketed, taking both their safety and effectiveness into account
- ensuring clinical trails meet robust standards and safeguard patient’s interests
- inspecting the quality of medicines as manufactured and distributed
- overseeing UK Notified Bodies that audit medical device manufacturers
- encouraging everyone to report suspected problems with both medicines and devices and then investigating these reports
- Investigating, and prosecuting where necessary, cases of non-compliance including advertising claims.
The Clinical Practice Research Datalink (CPRD)
- The Clinical Practice Research Datalink (CPRD) is the new English NHS observational data and interventional research service, jointly funded by the NHS National Institute for Health Research (NIHR) and the Medicines and Healthcare products Regulatory Agency (MHRA). CPRD services are designed to maximise the way anonymised NHS clinical data can be linked to enable many types of observational research and deliver research outputs that are beneficial to improving and safeguarding public health.
- CPRD is considered by many as the GOLD standard and its usage has resulted in over 890 clinical reviews and papers. The team at CPRD provides value-added services to the General Practitioners who contribute to the database and to the researchers who want to make use of this unique public health research tool.
The National Institute for Biological Standards and Control (NIBSC)
- The key function of NIBSC, the ‘National Institute for Biological Standards and Control ‘is the standardisation and control of biological medicines. They offer;
a) Biological reference materials and customized reference materials
b) OCABR testing and contract testing
c) Research collaborations
d) Advice and training
- NIBSC are the global leader in the field of biological standardisation, responsible for developing and producing over 90% of the International Standards in use around the world to assure the quality of biological medicines. NIBSC scientists have an international reputation for excellence in research and are widely consulted on issues of biological medicine safety and efficacy.
- The Institute is the UK’s Official Medicines Control Laboratory (OMCL), responsible for testing of biological medicines within the framework of the European Union. They have a particularly close relationship with the World Health Organisation (WHO) and is the leading WHO International Laboratory for Standards.
Its statutory duties are:
- To devise and draw up standards for the purity and potency of biological substances, to design appropriate test procedures and to advise on these matters;
- To provide or to arrange for the provision of laboratory facilities for the testing of biological substances, to carry out such testing, to examine records of manufacture and quality control of biological substances and to report on the results of such testing or examination;
- To prepare, approve, hold and distribute standard preparations of biological substances;
- To collaborate with the World Health Organisation, the European Pharmacopoeia Commission and other international organisations or bodies in relation to the establishment of standards for, the provision of standard preparations of, and the testing of biological substances;
- To carry out or arrange for the carrying out of research in connection with the functions referred to in a) to d) above; and
- To do all other things incidental or conducive to the discharge of the above functions.
- The delivery of these is of the utmost importance, since biological medicines include many of today’s most widely used medicines (vaccines, blood products and biotherapeutics), together with some of the most exciting prospects for the future.
- Rapid advances in science, in particular remarkable breakthroughs in genetics and biotechnology, are creating new opportunities and challenges at an unprecedented rate. At the same time, however, public expectation of medicine safety is increasing all the time, and trust in both manufacturers and public health authorities remains fragile.

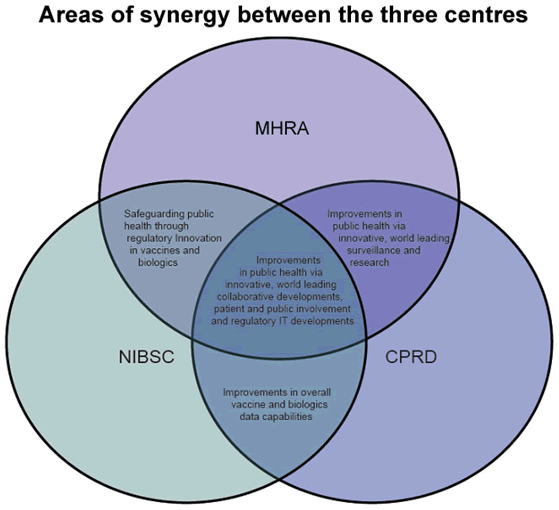
How being more integrated will bring benefits
As part of the same organisation, the three bodies will continue to provide all the same services and products as before, but in a more integrated way, helping to protect public health whilst bringing products to market reliably, efficiently, economically and safely through. The new organisation offers:
- Unrivalled scientific and regulatory expertise.
- A one stop shop for manufacturers within and outside Europe for advice on quality assurance, safety and regulation of biologicals.
- Unique ability to influence the development of science led regulation.
- Support for bringing innovation safely to market through the newly launched Innovation Office.
- Integrated approach to pharmacovigilance and risk management to deliver better public health.
What our stakeholders need to know
As a result of the changes we have updated the MHRA, NIBSC and CPRD identities to better reflect the expanded organisation and have introduced a Medicines and Healthcare Products Regulatory Agency family identity. You will start to see these being used on our materials in the coming months, from 1st April 2013.
More information on each of the centres is available on their website:
NIBSC http://www.nibsc.org/ (please note this has changed although your browser will redirect if you use the old URL)
CPRD http://www.cprd.com/ The MHRA and CPRD website addresses remain unchanged.