So where are we with malaria vaccines?
Mosquirix™ is the most advanced malaria candidate vaccine. Phase III clinical trials in infants were successfully completed in 2012 and it has since received a positive scientific opinion from the European Medicines Agency (2015). But there are more in the pipeline.
Developing a malaria vaccine is challenging, so manufacturers are adopting a number of approaches. One of the first things vaccine scientists need to consider is when to target the parasite.
The parasite’s life cycle is complex as it develops both within the mosquito and human host. It first enters the human host via a mosquito bite, then travels to the liver, where it replicates, before emerging and infecting red blood cells. Once the parasite is in the red blood cell, it undergoes cycles of replication, egress and re-invasion, infecting more and more red blood cells. Some parasites also undergo a transformation, remaining in the red blood cells for far longer and developing into the sexual stages of the parasite ready to be taken up by the mosquito during its next blood meal.
“Vaccines like Mosquirix™ protect against the early stages of the disease before the parasite begins to replicate in the blood. The idea is that by targeting this stage, you raise response against the parasite before it ever establishes and starts multiplying.”
“Another option is to target the parasite later, when it’s living within the patient’s red blood cells. As most clinical signs start to appear at this point, targeting this stage could stop the most dangerous symptoms of the condition from developing. Although targeting this stage wouldn’t block all infection, as the parasite would have already invaded the patient’s liver cells.”
“Transmission-blocking vaccines instead focus on preventing infection of the mosquito from humans and thus stop the parasite from being passed on from one person to the next. These vaccines present a really interesting dilemma as the vaccinated individual wouldn’t get any direct benefit from the vaccine, but they could prevent the spread of malaria through a population.”
As it progresses through its life cycle the parasite is continually changing, expressing different molecules on its surface. So once the stage has been selected, developers need to think carefully about the specific molecules on the parasite surface that could be vaccine targets. And this is where the issue of resistance again comes into play.
“At the moment the one vaccine we have targets a single malaria protein, sort of akin to a single malaria drug. Lots of different molecules on the parasite surface that have been identified as potential vaccine candidates have, over time, evolved to escape the human immune system and show huge amounts of genetic variation. So for any given protein that’s selected, there may be many different antigenic forms out there that are capable of causing an immune response.”
“By selecting only one antigenic form of a protein for a vaccine, there’s a risk that the vaccine form of the protein might disappear from the malaria population and the vaccine will eventually become ineffective”
With the emergence and spread of antimalarial resistance, current drug approaches look to combination therapies where more than one therapy is given to the patient at the same time to try and prevent resistance. Should a similar approach be adopted for vaccine development?
“Ultimately there’s all sorts of ways to approach vaccine design with respect to the life-stage targeted, numbers and genetic forms of proteins, or even whole parasites, and lots of different people are taking different routes.”
“Vaccines are being developed to cover all these bases, but the outstanding question is which ones are going to be the best? Should we target several antigens from one life stage, or one antigen from multiple life stages?”
“There might be a degree of overelaboration for some vaccine candidates that are targeting many different proteins from different parasite stages, but there are also potential concerns regarding resistance if all angles haven’t been covered at once. As it stands we don’t know enough about how they work to predict if they’ll prevent malaria, let alone if evasion is going to be a problem.”
Where we fit in
The team here at NIBSC are trying to design a number of tests that will help us to see how the parasite might evade pressure from the human immune system and give us an understanding of the protection each vaccine might offer.
“Currently, it’s very difficult to understand how effective vaccine candidates are outside of clinical trials in humans as we can’t recreate the immune system in the lab. So, in general, it’s hard to know how the current vaccine candidates are working.”
“In drug discovery, scientists will test the parasite’s ability to generate resistance to the drug of interest, by putting varying amounts of drug on the parasite and seeing what parasites survive to understand the basis of this resistance. It’s inconceivable that you’d go ahead with a drug without some sort of knowledge of potential resistance.”
“For biologics, we don’t have the same portfolio of things to test, or the range of assays to test them in. There’s several tests we can do in the lab to build up parts of the picture of the human immune response and my group is assessing and developing these to understand if they can be used to tell us how effective a vaccine might be and, also, how easily the parasite could change to evade it.”
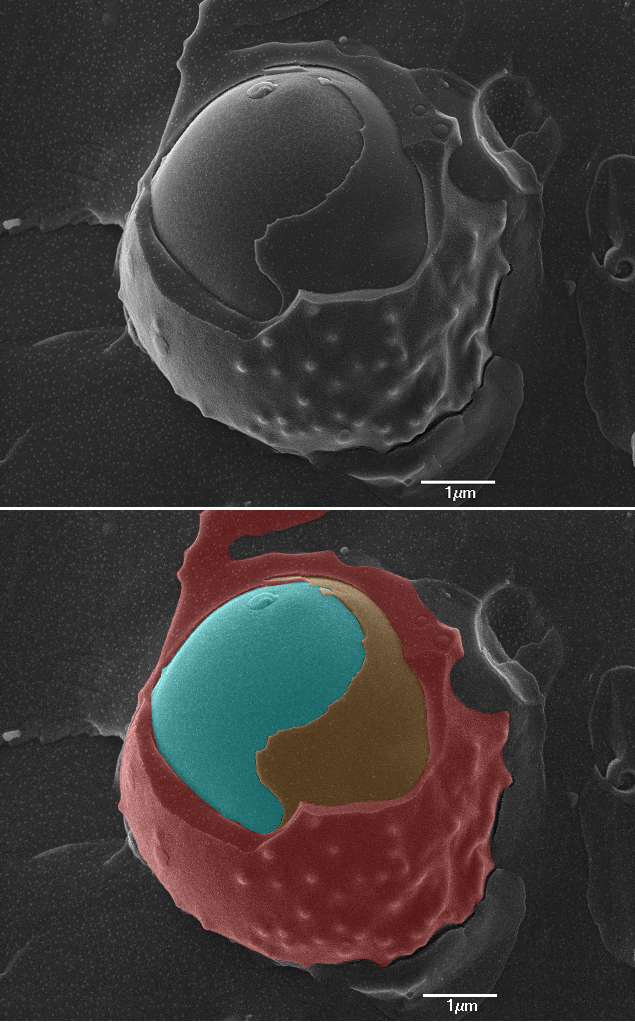
Cryoscanning Electron Microscopy (CryoSEM) image of a red blood cell (red) with a malaria parasite (cyan) within its parasitophorous vacuole (orange). Image courtesy of the Biological Imaging Group, NIBSC.