Blood cell and platelet immunology
Our group focuses on the immunology of red blood cells (erythrocytes), platelets (thrombocytes) and granulocytes to support blood transfusion, and the treatment of immunological conditions that relate to blood components. Our work covers:
-
Platelet and granulocyte immunology
Clinicians need to rely on the quality of the results coming from the laboratory when diagnosing and treating platelet and granulocyte-related immunological conditions. Our group help to standardise the field of platelet and granulocyte immunology providing reference materials and methods to support the identification of platelet and granulocyte-specific antibodies.
-
Red cell immunology
Our group supports the field of transfusion medicine and the generation of safe and effective blood products from plasma/blood donations. We provide standards and reagents that can be used in blood group serology, to assay levels of anti-D in sensitised individuals, to estimate potency of anti-D immunoglobulin products for prophylaxis and assay blood products for undesirable anti-blood group antibodies.
We also produce reference materials used for defining European Pharmacopoeial product specifications, and provide advice on methodology to make immunoglobulin products safer and more effective.
What we do
Standardisation
Platelet and granulocyte immunology
We provide WHO reference materials for platelet and granulocyte immunology. Our platelet immunology reference standards are minimum potency reagents consisting of freeze-dried, pooled human plasma and can be used to establish the sensitivity of an HPA antibody detection assay.
We also have an Anti-HNA-1a reference standard that can be used in granulocyte immunology to establish the sensitivity of an HNA antibody detection assay. This minimum potency reagent consists of freeze-dried, pooled human plasma.
We are currently looking for plasma or serum containing antibodies to either HPA-15a or -15b which could be pooled to make a minimum potency reagent. Please contact us if you are willing to donate material.
Standardisation of methods
We’ve produced several resources to improve test performance and support standardisation of methods used at NIBSC for the detection of platelet specific antibodies and for genotyping platelets.
Monoclonal Antibody-Specific Immobilization of Platelet Antigens (MAIPA) assay
Rapid MAIPA video
Rapid MAIPA protocol
Previous ‘consensus’ MAIPA protocol
Platelet Immunofluorescence Test (PIFT)
PIFT protocol
Single Specific Primer-Polymerase Chain Reaction (PCR-SSP)
PCR-SSP genotyping protocol
Platelet immunology quality scheme
The aim of the Platelet Immunology Quality Exercises organised by NIBSC between 1991-2017 was to help clinical laboratories concerned with detecting platelet-specific antibodies to achieve a high standard of performance by distributing coded samples for blind testing. The scheme had two parts, detecting and identifying platelet alloantibodies, and detecting HPA alleles by molecular techniques. Participants of the scheme were scored according to their testing performance and results distributed in an anonymized report.
Details of how laboratory performance is scored are available here
NIBSC Platelet Immunology Quality Scheme has now transferred to UK NEQAS for Histocompatability and Immunogenetics with 2017-A being the last exercise organised by NIBSC. Please direct any queries relating to previous exercises to lucy.studholme@nibsc.org.
Members of the NIBSC Platelet Immunology Quality Scheme wishing to transfer to the UK NEQAS scheme should complete the UK NEQAS HPA Genotyping and Platelet Antibody Analysis form.
For general information on UK NEQAS schemes, please visit neqas.welsh-blood.org.uk.
Nomenclature of human platelet antigens (HPA)
The nomenclature of HPA antigens and protein and genetic data are on the
Immuno Polymorphism Database hosted by The European Bioinformatics Institute (
EBI) which is part of
European Molecular Biology Laboratory (EMBL).
Red cell immunology
We have a range of reference preparations for use in blood group serology including WHO International Standards for anti-A, B and D blood grouping reagents, WHO International Reference Reagents for blood group genotyping methodology and CE-marked standards to assess anti-D and anti-C serum levels.
We’ve collaborated with the United Kingdom National External Quality Assessment Service Scheme for Blood Transfusion Laboratory Practice to produce World Health Organisation (WHO) reference reagents for consistent identification of donors and patients with high titre anti-A/B. These reagents are important for clinical practice and can help:
- minimise the risk of causing clinically significant haemolysis when blood components rich in plasma are transfused to patients of non-blood group O
- facilitate mismatched kidney transplants
- identify high titre anti-A/B plasma to exclude from pools intended for manufacture of blood products such as intravenous immunoglobulin (IVIG)
Control
Our work includes developing and implementing European Pharmacopoeia specifications, tests and reference reagents to make immunoglobulin products safer and more effective.
We’ve developed a competitive enzyme-linked immunoassay (EIA) for estimating anti-D potency of anti-D immunoglobulin products along with production of a lyophilised monoclonal anti-D reagent and a global standard for anti-D immunoglobulin.
In collaboration with the European Directorate for the Quality of Medicines & Healthcare (EDQM) and the Center for Biologics Evaluation and Research, Food and Drug Administration (CBER/FDA) we’ve also developed pharmacopoeial tests and specifications for anti-D, anti-A and anti-B in intravenous immunoglobulin (IVIG) to ensure IVIG products are safe and to harmonise global specifications. This includes:
- a direct haemagglutination test for anti-D in IVIG along with production of global reference reagents to control tests and define the pharmacopoeial limit
- a direct haemagglutination test for anti-A and anti-B in IVIG along with production of global reference reagents to control tests and define the pharmacopoeial limit
We routinely carry out these tests for Official Control Authority Batch Release (OCABR) of immunoglobulin products.
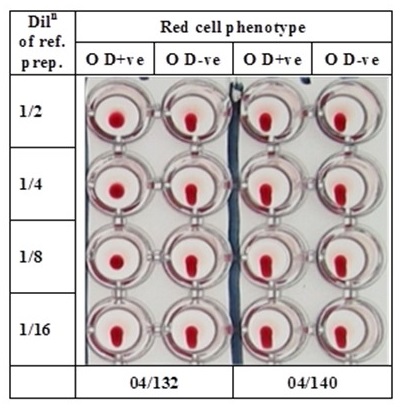
Reference reagents for controlling haemagglutination titrations for anti-D in IVIG. 04/132 defines the pharmacopoeial limit. Cell button = positive reaction; stream of cells = negative reaction.
In recent years, there has been increased reports of haemolysis associated with new generation IVIG products due to higher titres of anti-A and anti-B. We’ve supported investigation into this area by:
- identifying high titre anti-A – though within specification – in a batch of IVIG associated with renal failure in a patient
- producing data in support of our proposal to implement testing for anti-A and anti-B in IVIG as a European-wide OCABR test which was accepted and implemented in 2014
- sourcing an IVIG batch associated with haemolysis and made this available as an out-of-specification batch to help control haemagglutination tests performed by manufacturers and OMCLs, and as a research reagent in investigations of the factors contributing to haemolysis in certain patients.