Coagulation factors and inhibitors and antithrombotics
Bleeding is caused by insufficient clotting while thrombosis is caused by overt clotting. Clot formation or haemostasis is regulated by the balance of coagulation factors and inhibitors in the presence of blood cells within the vasculature. Factor VIII (FVIII) and factor IX (FIX) are the most common congenital bleeding disorders and are known as haemophilia A – which has an hereditary frequency of 1 in 10,000 – and haemophilia B with an hereditary frequency of 1 in 30,000. Patients suffering from congenital or acquired deficiencies of coagulation factors often require life-long treatment with coagulation factor therapeutics. Thrombophilia patients with congenital deficiencies of plasma coagulation inhibitors such as antithrombin and protein C are treated with antithrombin and protein C concentrates respectively. For acquired thrombotic disorders, heparin, low-molecular-weight heparins or oral anticoagulants are used to prevent thrombosis. These disorders include, but are not limited to:
- lupus anticoagulants – which affects 1-5% of the healthy population and accounts for 20% of recurrent thrombosis in young adults
- deep vein thrombosis which has been diagnosed in 10-40% of medical or general patients and 40-60% of orthopaedic surgical patients as an iatrogenic complication

|
Haemostasis results from a balance of procoagulant and anticoagulant factors. Increase in activity or amount of procoagulant factors increases the risk of thrombosis. Absence of procoagulant factors or decrease in anticoagulant factors could lead to bleeding disorders.
|
What we do
Our laboratory has the control and standardisation responsibilities for antithrombotics, coagulation factors and inhibitors. An active research and development programme is in place to underpin the control and standardisation remits which aid correct diagnosis and safe and effective treatment of bleeding and thrombotic disorders.
Standardisation
We produce World Health Organisation (WHO) International Standards, pharmacopeial standards, British working standards and NIBSC reagents.
In the diagnostics area these are:
- antithrombin
- protein C
- protein S
- FII, IX, X, XI, XII
- β-thromboglobulin
- platelet factor 4
- lupus anticoagulant
- genetic reference panel for haemophilia A intron 22 inversion
- genetic reference panel for FV Leiden
- genetic reference panel for Prothrombin G20210A
In the therapeutics area these are:
- antithrombin
- protein C
- FII, IX, X, XI
- FIXa
- FXIa
- potency standard for unfractionated heparin
- potency standard for low-molecular-weight heparin
- molecular-weight calibrant for low-molecular-weight heparin
In the reference reagents area these are:
- recombinant FIX
- reference plasma for thrombin generation tests – TGT
- tissue factor for global tests – TGT, TEG, ROTEM
|
Recent Highlights in Standardisation:
Contact activation factors XI and XII
Factors XI and XII are part of the intrinsic coagulation pathway and can be activated by contact with surfaces or agents, resulting in initiation of the coagulation cascade. Though clinical symptoms are absent or mild, factor XII and factor XI deficient patients often require monitoring and purified factor XI therapeutics are licensed as replacement therapy for congenital and acquired FXI deficiency. Factor XII is important in certain inflammatory and thrombotic diseases and its inhibition provides an attractive opportunity for new therapies to be developed. In order to underpin both clinical monitoring of patients and to aid standardisation of assays for new therapeutics, NIBSC has developed the 1st International Standard for factor XII, plasma. Both this and the International Standard for factor XI, plasma, have assigned values for functional activity and antigen, which will support and harmonise factor XI and XII assays and related therapeutics.
|
Control
We perform National Control Laboratory Batch Release of high purity FIX, Prothrombin Complex Concentrates, FX and Antithrombin therapeutics in compliance to ISO/IEC 17025, with independent accreditation by the United Kingdom Accreditation Service (UKAS).
Research and development
Our activities are:
New generation FVIII and FIX therapeutics
Replacement therapy has entered a new era with the introduction of ‘extended half-life’ products specifically designed using chemical and genetic modifications to reduce the frequency of infusion and facilitate control of trough levels:
- pegylation
- fusion with the Fc portion of the immunoglobulin molecule
- intra-chain covalent bonds
In many cases these modifications to the FVIII or FIX molecule result in methods-related potency discrepancies when measured relative to the WHO IS concentrates and this raises the possibility of alternative approaches to potency labelling. The Section is ideally placed to support a harmonised approach to potency labelling and several initiatives have been explored:
- single laboratory and multi-centre testing of new products:
- relative to the WHO International Standard Concentrate to identify methods-related potency labelling issues
- relative to the WHO International Standard Plasma to anticipate issues of post-infusion testing
- collaboration with regulators, pharmacopoeias and manufacturers to promote a harmonised approach to potency labelling through:
- elaboration of recommendations and guidelines
- presentations at scientific meetings and workshops
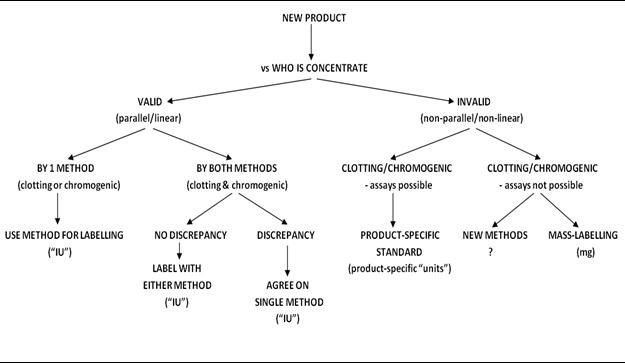
Decision tree for potency labelling of new FVIII therapeutics (Hubbard et al J Thromb Haemost 2013; 11, 988)
For anticoagulant and non-anticoagulant activities of heparin and related polysaccharides:
|
Goals
- development of in vitro test methods to identify adulterants in clinical heparin products
- comparison of the biological activities of porcine and bovine heparin
- elucidation of structure and function relationship for pro- and anti-inflammatory activities
|
Antithrombotic drugs are agents which are used to prevent or limit blood coagulation from occurring. The prime purpose for these agents is in situations where blood must maintain fluidity such as during surgery or dialysis. Other situations are when there is a low level procoagulant state which requires some form of therapy to stop thrombosis formation which may result in a heart attack or stroke. Arguably the most widely used drug in the world is the antithrombotic unfractionated heparin (UFH), whose use ensures blood fluidity during and after surgical procedures. Low molecular weight heparin (LMWH), which is a derivative of unfractionated heparin, has more predicative pharmacokinetics and is used in treat such conditions as pulmonary embolism and deep vein thrombosis. In situations of an increased thrombotic state, such as cancer, LMWH is the treatment of choice.
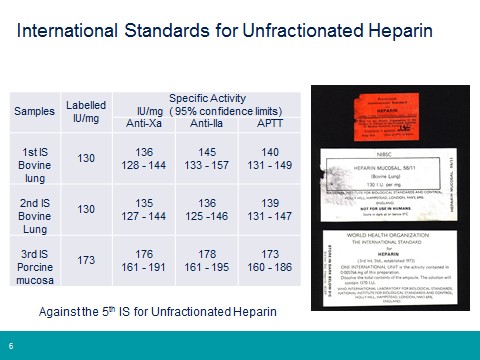
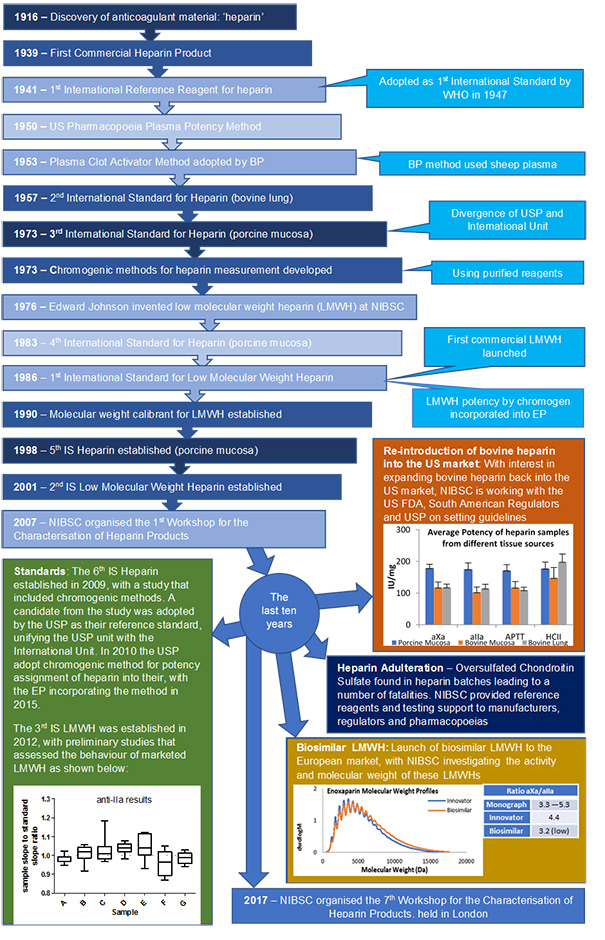
NIBSC has a long history in the heparin field (see below), from the establishment of the 1st International Reference Preparation for unfractionated heparin in 1941, to the invention of low molecular weight heparin in 1976 and supporting regulators and pharmacopoeias in response to the heparin contamination issue of 2007/08. Throughout all the years that heparin has been used as a clinical medicine the WHO International Standard has ensured continuity of unitage for all heparin products. Each successive standard is value assigned against the previous material in an international multicentre study, meaning the current 6th IS (established in 2009) is fully traceable to the very first heparin standard. This approach has also been used for both the low molecular weight heparin standard and the low molecular weight heparin for molecular weight calibration.
 |
|
Continuity of International Unit for Unfractionated Heparin
|
Our current areas of interest in the heparin field are:
- development of in vitro test methods to identify adulterants in clinical heparin products
- comparison of the biological activities of porcine and bovine heparin
- investigations into the activities/molecular weights of biosimilar LMWH
- elucidation of structure and function relationship of non-anticoagulant heparin and related materials
For procoagulant activities of immunoglobulin products:
|
Goals
- develop and refine assay methods for procoagulant activities of immmunoglobulins
- develop and produce reference preparations that will help to promote global harmonisation of these assay methods
|
In 2010, increased thromboembolic events (TEE’s) after administration of immunoglobulin products led to the recall of a number of batches from the market.
Investigation by stakeholders into the cause revealed that due to a small change in manufacturing process; activated coagulation factor FXI (FXIa) was co-purifying with the immunoglobulin. Since then steps have been taken by the manufacturers of immunoglobulin products to remove this process related impurity.
However, the measurement of procoagulant activity in these products continues to be a challenge due to a lack of harmonised protocols and appropriate reference materials leading to poor intra- and inter-laboratory agreement. In addition, there are no agreed limits and specifications for these tests.
Our involvement in the harmonization of assay methods and production of reference materials:
- 2 NIBSC panels of freeze dried immunoglobulin with different levels of procoagulant activity
- Establishment of the International Reference Reagent for Activated Coagulation Factor XI, 11/236
- Establishment of the International Standard for Activated Coagulation Factor XI, 13/100 (link to catalogue)
- Formation of a Global Working Group (GWG) in 2014 for the Measurement of Procoagulant Activity in Immunoglobulin products- membership from the EDQM, USFDA/CBER, USP and NIBSC. Initiatives include:
- 2015 survey of immunoglobulin manufacturers and Official Medicines Control Laboratories (OMCLs) current laboratory practices for the measurement of procoagulant activity in immunoglobulin products. (Link to survey report?)
- Stakeholder forum sponsored by the Plasma Protein Therapeutics Association (PPTA). Washington DC, September 2016. (Link to meeting summary?)
- Formation of small study groups to identify critical steps within individual assay methods
- Development of a panel of immunoglobulin products with different formulations/excipients to use as collaborative study material and available for kit manufactures to aid assay development
|
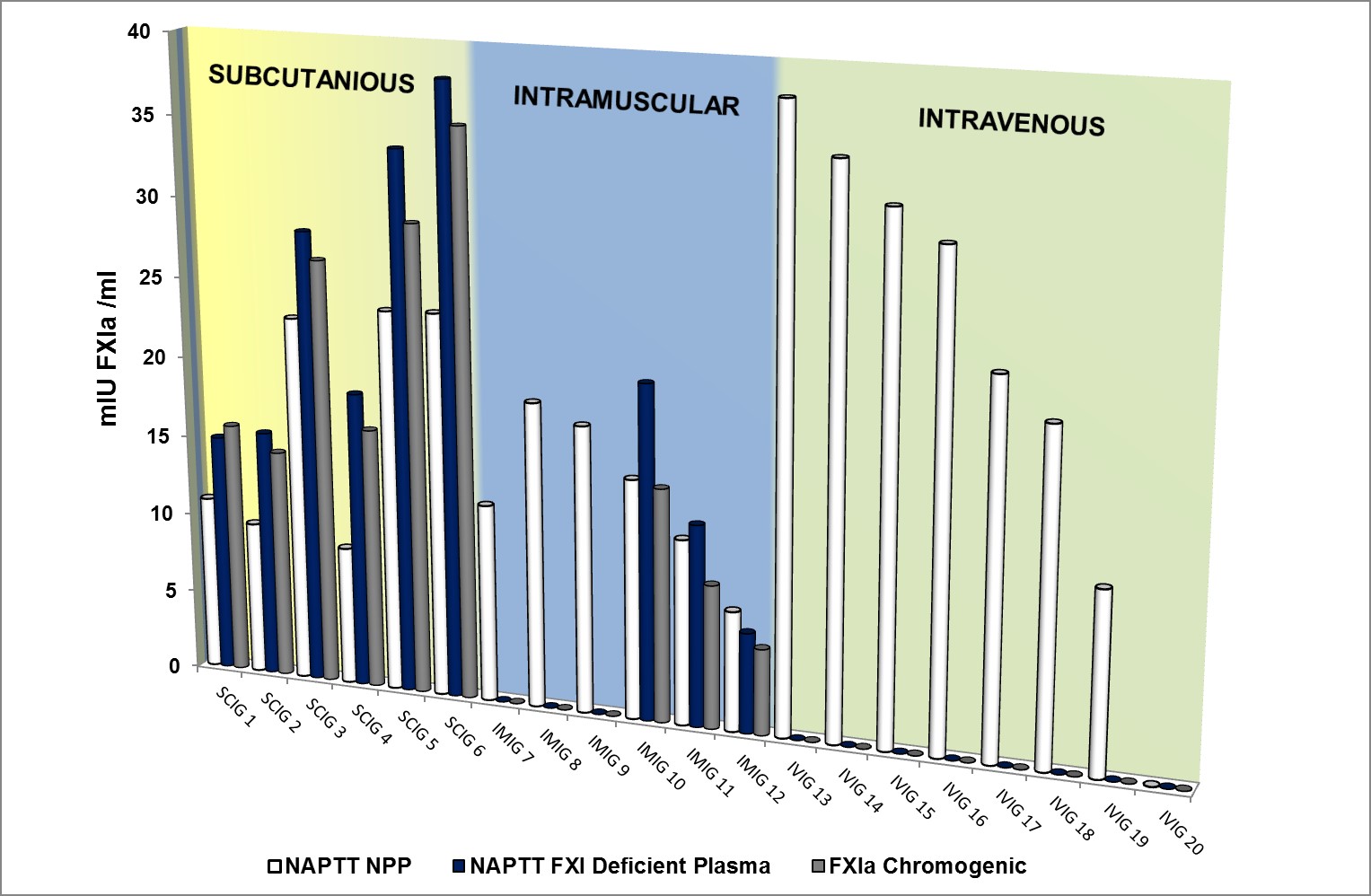
Procoagulant activity expressed as mIU FXIa /ml in 20 commercially available immunoglobulin (IG) batches from 6 manufacturers (consisting of 6 subcutaneous IGs – yellow panel, 6 intramuscular IGs – green panel and 8 intravenous IGs- purple panel). Results show method related discrepancies in FXIa activity when measured using 3 different methods; FXIa functional chromogenic assay (grey bars), Non Activated Partial Thromboplastin Time (NAPTT) using FXI deficient plasma (blue bars) and NAPTT using pooled normal plasma (white bars).
|