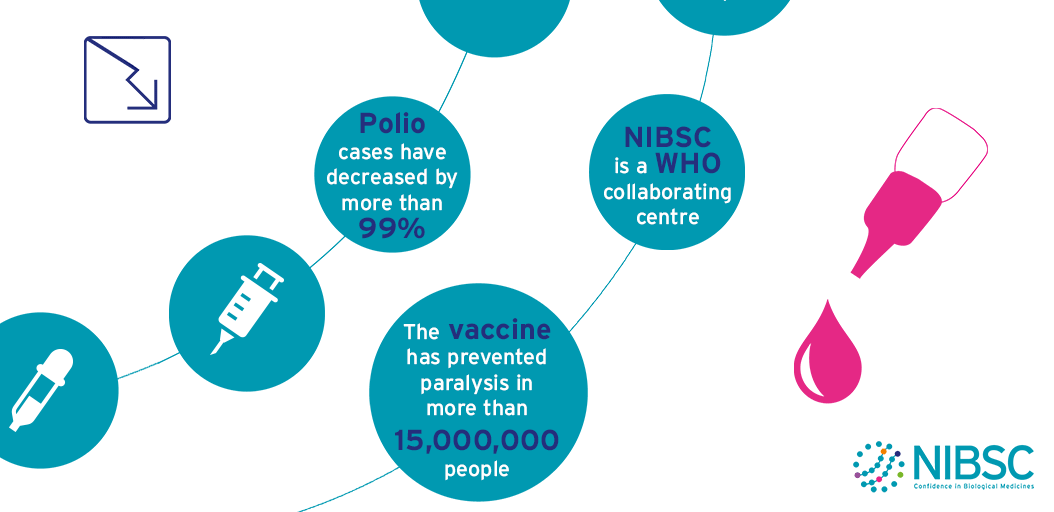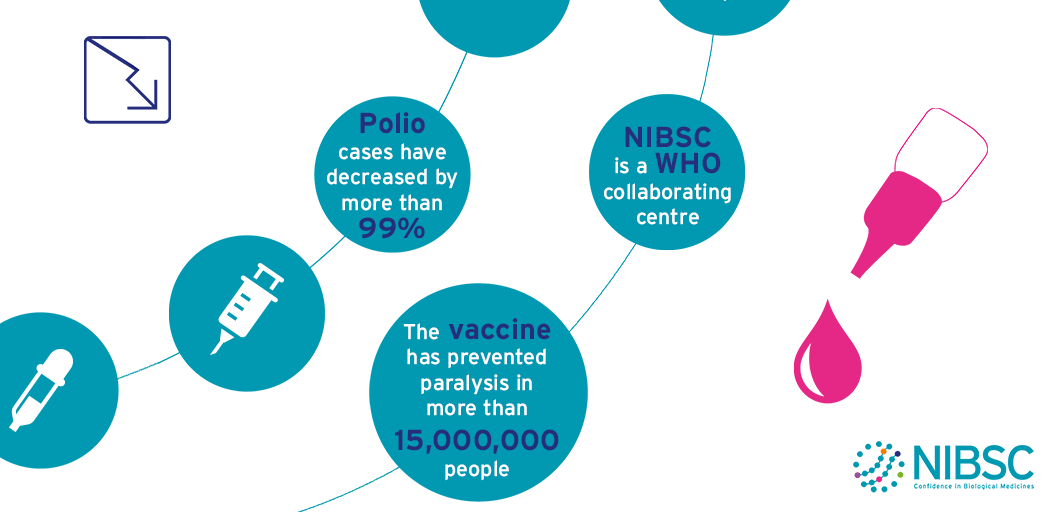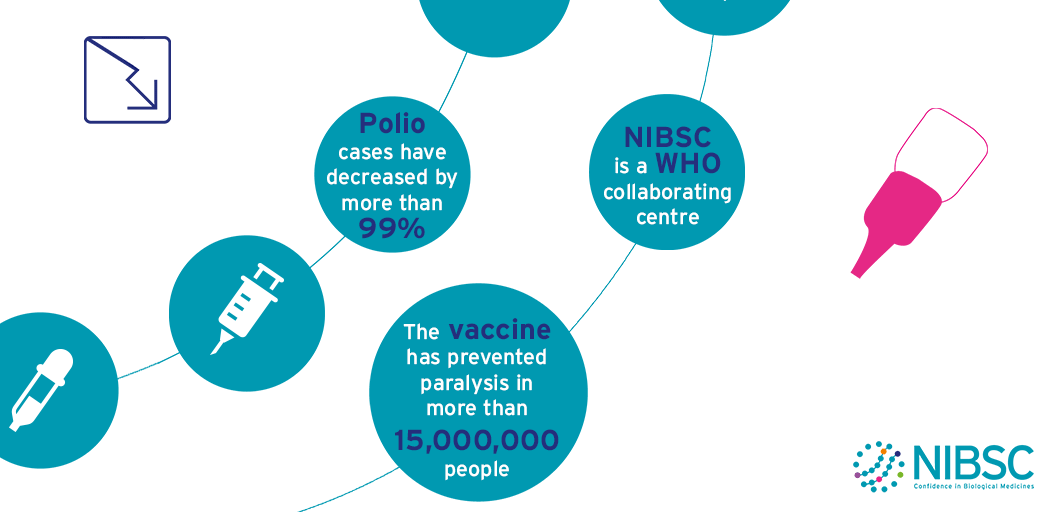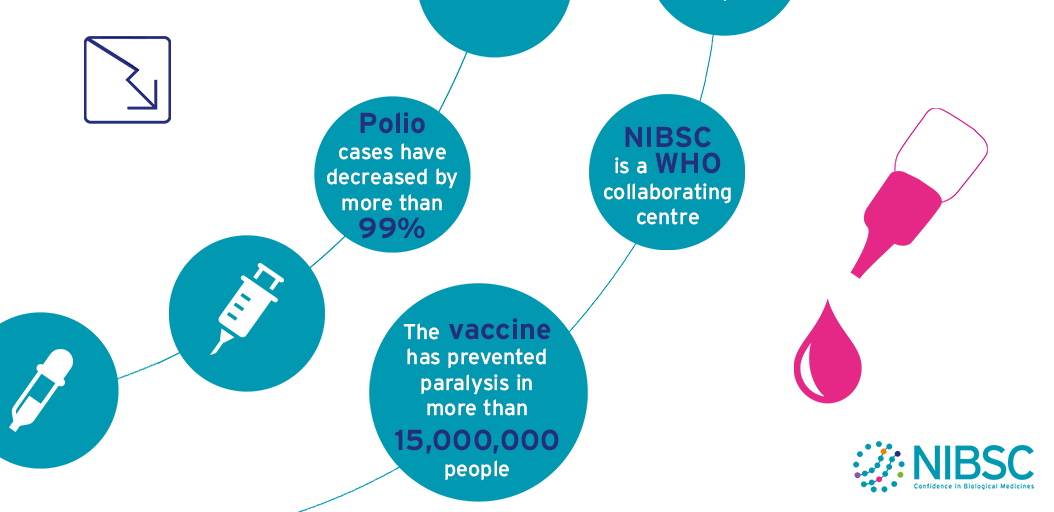
Polio
The aim to eradicate polio around the world has some major issues to overcome.
Wild-type poliovirus is endemic in three countries, and a few cases of polio are imported into previously polio-free areas every year.
Meanwhile, a small number of outbreaks due to vaccine-derived poliovirus (VDPV) are still being reported.
The World Health Organisation (WHO) and its partners have drawn up an ambitious strategic plan to finish the eradication process and sustain global polio-free status after the process is complete.
This plan includes step changes in vaccination strategies, enhanced surveillance and the global containment of poliovirus laboratory samples. As ever, scientific research and laboratory analyses will be needed to support these activities, particularly at critical decision stages.
For many years NIBSC’s scientists have contributed to the scientific knowledge of poliovirus basic biology that has been the basis for establishing laboratory assays and animal and cell culture models for poliovirus. In particular, early pioneer work helped identify the molecular basis for:
- attenuation of OPV strains
- antigenicity and immunogenecity of live and inactivated poliovirus strains
- replication and evolution of vaccine and wild-type poliovirus in humans
Because of this we have established great expertise in a wide array of polio assays and have assembled one of the world’s most comprehensive collections of critical polio laboratory reagents such as cell lines, virus isolates, vaccine reference preparations and monoclonal antibodies.
Read more about how NIBSC has been leading important research to support the eradication of polio for the last 40 years.
Control and surveillance
Our group has two main roles which provide essential support to the World Health Organisation’s (WHO) Global Eradication initiative (GPEI).
First, we provide surveillance for poliovirus to confirm its presence or absence in clinical samples and to characterise poliovirus isolates from surveillance activities.
Secondly we carry out quality control of poliovaccines to assess the quality of live attenuated oral polio vaccine (OPV) and inactivated polio vaccine (IPV) by establishing norms and standards, testing products for batch release and investigating adverse events.
These activities are framed within the terms of reference of NIBSC’s role as a WHO Collaborating Centre for Reference and Research on Poliomyelitis which include:
- laboratory testing clinical samples and poliovaccines
- production, establishment and distribution of International Standards and working reagents
- training laboratory staff and managers
- research to support endgame strategies for the GPEI
- assisting manufacturers and national regulatory authorities during the implementation, validation and proficiency testing of the different techniques in their laboratories
- independent technical expertise and advice that contributes to the WHO recommendations and European Directive for the Quality of Medicines (EDQM) monographs for the production and quality control of poliovaccines
NIBSC is one of the 7 WHO Global Specialised Laboratories (GSLs) that sit at the top of the three-tiered structure of 146 polio network laboratories (Specialised, Regional and National). In this role NIBSC is accredited by WHO to perform virus isolation, intra-typic differentiation by real-time RT-PCR and nucleotide sequencing to definitively identify poliovirus isolates from surveillance activities. This information is essential to establish the temporal and geographical transmission pathways of poliovirus circulation.

NIBSC is also accredited by the United Kingdom Accreditation Service (UKAS), EDQM and WHO to perform testing for the batch release of poliovaccines in the UK, Europe and globally. We provide a global resource to support manufacturers and national regulatory authorities to produce safe and effective vaccines. At present, NIBSC is the only institution in the world capable of providing support in all the technically complex laboratory techniques used for the evaluation of OPV and IPV products, such as the Mutant Analysis by polymerase chain reaction (PCR) and Restriction Enzyme Cleavage (MAPREC) molecular test and the TgmNVT test in transgenic mice.
Our control tests:
- Batch release for OPV:
- potency test in cell culture
- MAPREC test
- transgenic mouse neurovirulence test (TgmNVT)
- Batch release for IPV:
- ELISA test
- rat test
- immunisation/challenge test in transgenic mice
- Polio surveillance:
- virus isolation in cell culture
- intratypic differentiation by cell culture neutralization
- real-time RT-PCR for intratypic differentiation
- nucleotide sequencing of poliovirus isolates
- cell sensitivity assay
- real-time PCR for cell authentication
Research
Research underpins all our standardisation, testing and surveillance activities. Current research interests include:
- studies to assess the quality and efficacy of existing and new poliovaccines
- using next generation nucleotide sequencing techniques to analyse virus population dynamics and poliovaccines
- characterisation of molecular processes responsible for poliovirus inactivation
Much of our work in these areas is aimed at contributing to the 3 Rs (replacing, refining, reducing) strategy of working with laboratory animals. We have important collaborations with laboratories and institutions in the UK and around the world such as Imperial College London in the UK, the National Institute for Infectious Diseases in Japan, the Food and Drug Administration in USA and the National Institutes for Food and Drug Control in China.
Highlights from previous research include:
- first description of a poliomyelitis outbreak due to VDPV strains which followed a vaccine clinical trial – several VDPV outbreaks due to OPV-derived strains have been identified since then and this is now regarded as one of the biggest threats to the completion and sustainability of the GPEI
- first isolation from humans of an heterotypic capsid recombinant poliovirus – a rare phenomenon that leads to viruses with altered antigenic structures
- several studies on the evolution of poliovirus vaccine strains in healthy and immunodeficient individuals identifying differences in population dynamics and mutation profiles
- phylogenetic analysis of wild-type poliovirus isolates from Ghana which helped understand the impact of surveillance activities and OPV campaigns on the circulation of wild-type poliovirus
- analysis of the antigenic and neurovirulence properties of wild-type poliovirus strains from sewage samples in Israel where widespread circulation occurred despite very high immunisation coverage with IPV – our results indicate that IPV is efficient at protecting against paralytic disease but inefficient at preventing circulation of wild-type poliovirus
image: Dunn G, et al. (2015) Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative. PLoS Pathog 11(8): e1005114. doi: 10.1371/journal.ppat.1005114