SoGATS Workshops
Meetings of the SoGAT group are typically held every 1-2 years. Participation is encouraged from blood and clinical laboratories, manufacturers of diagnostic assays and quality control reagents, providers of external quality assessment schemes, and regulatory and public health authorities. For more information or to sign up to our mailing list please contact us at sogat@nibsc.org
SoGATS 2025:
A three-day meeting was held at the MHRA Science Campus in South Mimms, UK,on 24th-26th September 2025. Highlights included:
- Updates from WHO Collaborating Centre’s on progress with new/replacement reference materials
- Discussions about the challenges associated with preparing commutable reference materials for infectious disease diagnostics, and the nature of target analytes in clinical samples
- Updates on the use of digital PCR in support of pandemic preparedness.
- Discussions about the provision of reference materials for High-Throughput Sequencing for Metagenomics and genetic variance/drug resistance monitoring.
This hybrid meeting was attended by over 100 delegates working in infectious disease diagnostics.
WHO reference materials for NAT
World Health Organisation (WHO) biological reference materials provide a common set of standards which are used to ensure the quality of biological medicines worldwide.
International standards are manufactured according to WHO guidelines and are considered to be the ‘gold standard’ against which regional, national and international laboratories and manufacturers calibrate their own working standards.
They are calibrated in units of biological activity (International Units, IU) which are assigned following extensive studies involving multiple international laboratories. They are then formally established following review by the WHO Expert Committee on Biological Standardisation (ECBS) at its annual meeting in October.
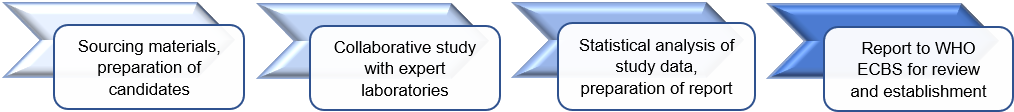
Recently established WHO reference materials for NAT, antigen or serology for infectious diseases:
| WHO reference material | Product code | Date of establishment | WHO ECBS report |
|---|
| 1st WHO International Standard for HIV-1 p24 Antigen |
22/230 |
March 2024 |
WHO/BS/2024.2470 |
| 1st WHO International Reference Panel for Lassa virus for NAT |
21/112 |
March 2024 |
WHO/BS/2024.2468 |
| 1st WHO International Standard for antibodies to Marburg virus for binding assays (human serum) |
23/146 |
October 2024 |
WHO/BS/2024.2482 |
Further information on these NAT and serology standards, and others, is available on the WHO website.
WHO International Standards for NAT, antigen, or serology for infectious diseases in development:
| Proposed WHO Reference material | WHO Collaborating Centre | Expected completion |
|---|
|
NAT:
|
|
|
|
1st International Standard for Aspergillus fumigatus DNA for NAT
|
MHRA/NIBSC
|
2028
|
|
1st International Standard for Mpox DNA for NAT
|
MHRA/NIBSC
|
2027
|
|
1st International Standard for Influenza AB for NA
|
MHRA/NIBSC
|
2028
|
|
2nd International Standard for Human Cytomegalovirus DNA for NAT
|
MHRA/NIBSC
|
2027
|
|
2nd International Standard for Hepatitis E Virus RNA, for NAT
|
PEI
|
2029
|
|
|
|
|
|
Antigen:
|
|
|
|
1st International Standard for Aspergillus fumigatus galactomannan
|
MHRA/NIBSC
|
2028
|
|
4th International Standard for Hepatitis B Surface Antigen
|
MHRA/NIBSC
|
2026
|
|
|
|
|
|
Serology:
|
|
|
|
Replacement WHO International Standard for Rubella IgG
|
MHRA/NIBSC
|
2027
|
|
1st International Standard for Dabies bandavirus (SFTS) antibodies
|
MHRA/NIBSC
|
2028
|
|
1st International Standard for Antibodies to Crimean-Congo haemorrhagic fever virus
|
MHRA/NIBSC
|
2026
|
|
3rd International Standard for anti-Hepatitis A Immunoglobulin
|
MHRA/NIBSC
|
2026
|
|
2nd International Standard for Human Syphilitic Pasma IgG and IgM
|
MHRA/NIBSC
|
2026
|
|
2nd International Standard for anti-Varicella Zoster Virus Immunoglobulin
|
MHRA/NIBSC
|
2026
|
|
1st International Standard for antibodies to Mpox virus
|
MHRA/NIBSC
|
2026
|
|
3rd WHO International Standard for anti-Hepatitis B Surface Antigen Immunoglobulin
|
MHRA/NIBSC
|
2027
|
History of SoGATS
Blood virology:
In 1994, the European Plasma Fractionation Association (EPFA), in conjunction with NIBSC, held a workshop to discuss the potential applications of NAT; from the detection of blood borne viruses in blood donation screening, to the production of “clean” plasma derived products. The meeting concluded that there was a need for standardization of NAT in the blood safety field, and that this warranted the formation of a group dedicated to standardization of NAT-based assays for blood borne viruses.
The 1st meeting of the International Working Group on the Standardisation of Genomic Amplification Techniques (SoGAT) for the virological safety testing of Plasma and Blood derived Products took place in April 1995. The group met to discuss standardisation techniques for blood viruses such as HCV, HBV, B19 as well as HIV. The rapid development of commercial assays for NAT and the appreciation of the scope of this new technology by many diagnostic and research laboratories, led to a decision that there was a need for reference materials for blood borne viruses.
Since 1994 the group have been paramount in the development of International Standards, reference panels and working reagents for HIV-1 RNA, HAV RNA, HCV RNA, HBV DNA and B19V DNA. Today, the group continues to support the development of new and replacement standards and reference panels and exchange information relevant to the scientific, technical and regulatory aspects of NAT.
Clinical diagnostics:
Using the experience and knowledge gained by the SoGAT group focusing on blood virology, a new SoGAT group was established to address the area of clinical virology, which remains largely unstandardised. The International SoGAT Clinical Diagnostics Working Group is focused on the standardisation of NAT assays used in the diagnosis of clinical pathogens. The group was established in parallel to the SoGAT Blood Safety Working Group in 2008 and the first meeting took place at NIBSC in June of that year. The group has supported the development of International Standards for HCMV, EBV, BKV, JCV, AdV and HHV-6 viruses for NAT, and new projects for HSV1/2, influenza A and B, RSV and enterovirus NAT standardisation.
Since 2013, the SoGAT blood and clinical groups have met at an annual joint workshop. Participation is encouraged from blood and clinical laboratories, manufacturers of diagnostic assays and quality control reagents, providers of external quality assessment schemes, and regulatory and public health authorities.
SoGATS Aims:
- Lead the development of WHO Reference Reagents and International Standards suitable for NAT and serological infectious disease assays (for screening of blood donations, plasma pool testing and diagnostics)
- Provide guidance on the preparation of external control materials calibrated against the WHO International Standards to be included in each run to ensure the reliability of the results
- Understand the relationship between clinical samples and the WHO ISs
- Promote standardisation of NAT and serological assays through inter-laboratory comparison studies or collaborations with EQA providers
- Provide a forum for the exchange of information to develop standards to support new technologies
- Provide a forum to react quickly to the standardisation needs of emerging or re-emerging pathogens.
Publications
The following articles highlight the work and successes of SoGAT or provide further information on the standardisation of NAT assays:
SoGAT - 25 years of improving the measurement of nucleic acids in infectious disease diagnostics (a review). Morris C, Govind S, Fryer J and Almond N. Metrologia. 2019 doi:10.1088/1681-7575/ab2aa3
WHO manual for the preparation of secondary reference materials for in vitro diagnostic assays designed for infectious disease nucleic acid or antigen detection: calibration to WHO International Standards. WHO Technical Report Series 2017. Geneva, Switzerland:WHO 2017; 1004, 389-455.
Standardisation of nucleic acid amplification assays used in clinical diagnostics: a report of the first meeting of the SoGAT Clinical Diagnostics Working Group. Fryer JF, Minor PD. J Clin Virol. 2009 Feb;44(2):103-5. doi: 10.1016/j.jcv.2008.11.007.
Stay up to date
For more information or to sign up to our mailing list please contact us at sogat@nibsc.org.