Monoclonal Antibodies
Monoclonal antibodies (mAbs) are complex proteins that specifically react with sites, or epitopes, on target molecules. They have become important research tools and are also a highly successful class of biological drugs. The therapeutic monoconal antibody field continues to expand and mAbs are already an integral part of healthcare systems around the world with products available to treat cancer, inflammation, autoimmunity and infectious disease.
With the expiry of some patents on early ‘blockbuster’ drugs, drug manufacturers are increasingly interested in developing ‘biosimilars’. Some biosimilar mAbs have already been approved for use and the number of available biosimilar mAbs is expected to continue to grow over the next few years.
What we do
Our work in this area focuses on:
We also participate in expert working groups at European and international level (EDQM and WHO).
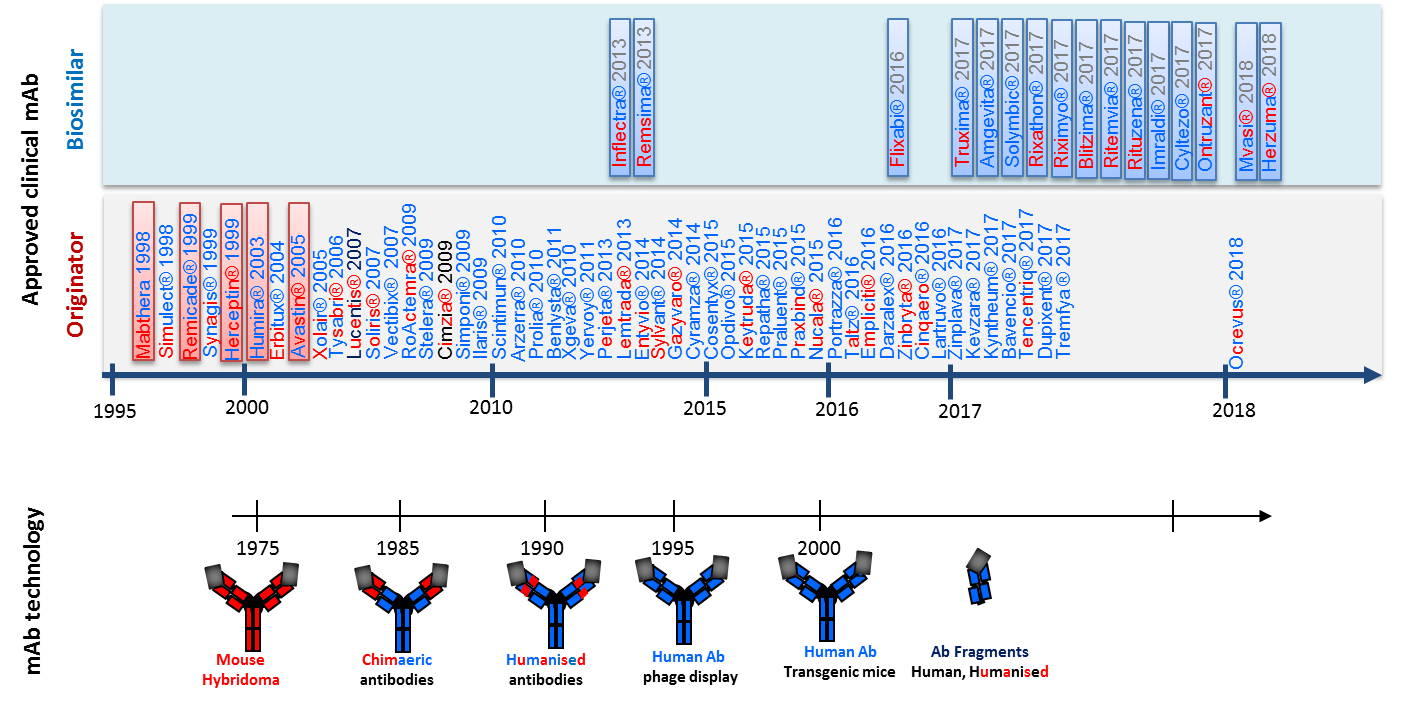
Biotherapeutic monoclonal antibody products authorized in Europe and related advances in antibody technologies
Standardisation
Monoclonal antibody standards for bioassays and reference reagents
MAbs are complex and heterogeneous biological medicines. Current regulatory approval of biosimilar mAb products involves exhaustive comparability studies with the originator product (reference medicinal product) to demonstrate biosimilarity.
With an increase in the number of biosimilars available and under development, there is a need for primary reference standards for biological activity. These preparations are stable and publically available standards that further contribute to ensuring product consistency, quality, safety and efficacy. Endorsed by WHO, we are committed to developing international standards (IS) for a number of mAbs including rituximab, trastuzumab, cetuximab, infliximab, adalimumab and bevacizumab.
International multicentre collaborative studies have already shown the benefits of these reagents to harmonise potency data for Infliximab and Rituximab. By defining international units (IU) of bioactivity, these standards can support the performance and calibration of bioassays and in-house standards over long periods of time.
 The distinct roles of the WHO international standard and the reference medicinal product
The distinct roles of the WHO international standard and the reference medicinal product
Pipeline of monoclonal antibody (mAb) standards
| Name | Predicted completion date | Status |
|---|
| Cetuximab |
2019/2020 |
New IS |
| Trastuzumab |
2019/2020
|
New IS |
To donate materials for reference standards or critical reagents or to participate in collaborative studies for the current pipeline standardisation programs please contact enquiries@nibsc.org.
Research
Bioassay development
The structural characteristics of monoclonal antibodies have been exploited, and in many cases carefully engineered, to develop biotherapeutic monoclonal antibodies with enhanced efficacy and multiple mechanisms of action. This can include blocking or neutralising a target by interacting with its cognate ligand, as well as stimulating (agonist) or inhibiting (antagonist) a biological receptor pathway. Many antibodies particularly in cancer therapy utilise their Fc effector functions, i.e. ADCC (Antibody dependent cell-mediated cytotoxicity) or CDC (Complement dependent cytotoxicity) as their primary mechanism of action.
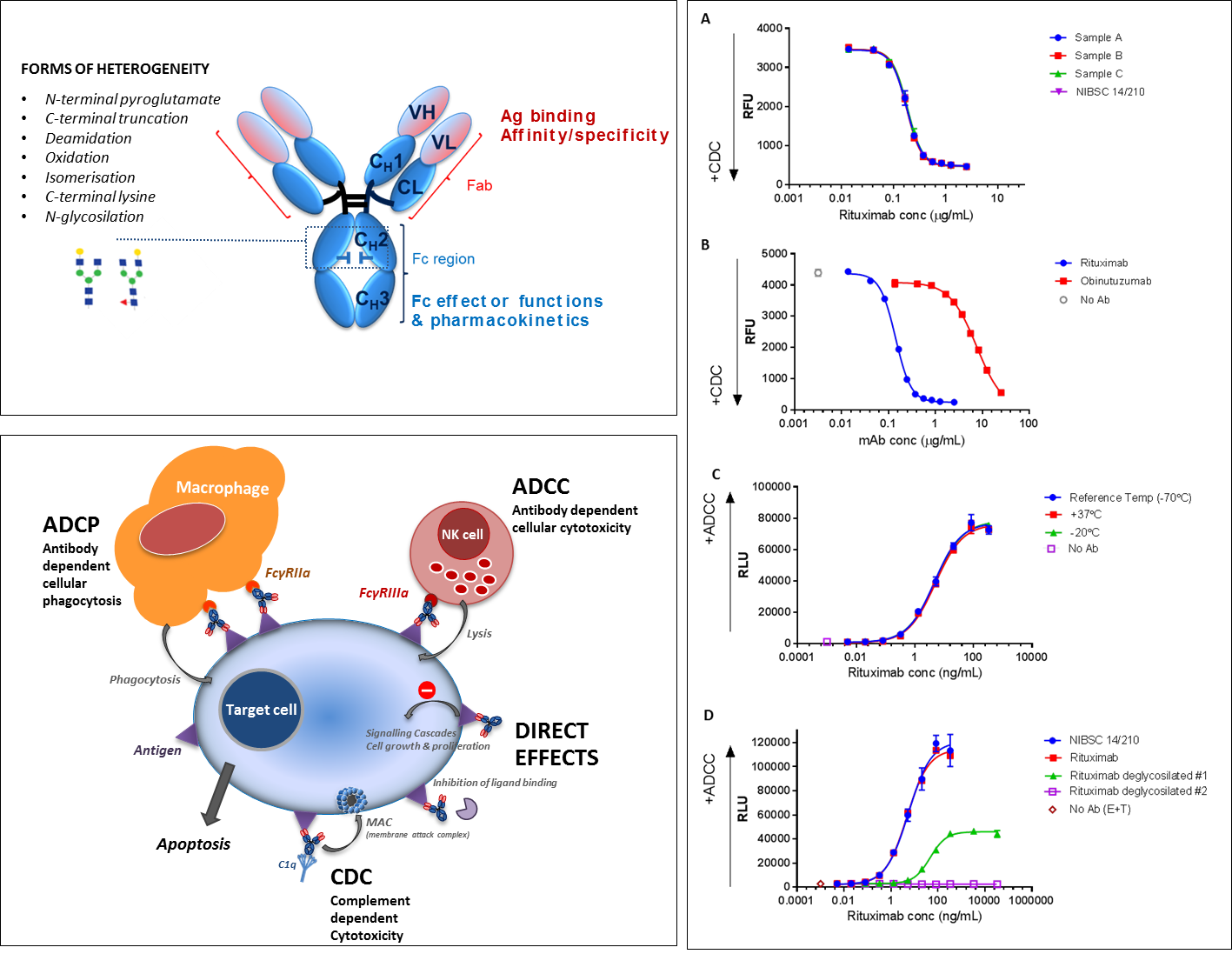
The structure of IgG and typical forms of monoclonal antibody (mAb) heterogeneity (post-translational modifications and degradation) and mechanisms of action. The mechanism of action of biotherapeutic mAbs are modulated by their structural characteristics and isotype form. A and B illustrate the CDC activity of 3 rituximab preparations (samples A-C) and the rituximab international standard (NIBSC 14/210) and the relative CDC activity of rituximab and obinutuzumab, respectively. Panel C shows the ADCC activity of the NIBSC 14/210 rituximab IS preparation during thermal degradation stability studies and panel D shows the relative ADCC activity of two deglycosilated rituximab variants.
We investigate structure-function relationships and mechanism(s) of action of monoclonal antibodies by developing relevant in vitro bioassays. We also aim to better understand the impact of product heterogeneities on quality, safety and potency in order to support the testing and development of consistent, safe and efficacious biotherapeutic mAbs.
Monoclonal Antibody Engineering and purification
Traditional methods used to produce monoclonal antibodies rely on the immunization of laboratory animals and the selection of immortalized clonal hybridoma cells. This process is laborious, requires use of animals and success is dependent on the ability of the immune system to mount an effective immune response to the chosen antigen.The advent of molecular biology and phage display technology has allowed new approaches to re-capitulate the
in vitro immune response, reducing and even eliminating the need for animal immunisation. We currently use these techniques to:
- Convert hybridoma-derived antibodies into recombinant antibodies
- Isolate antigen specific single domain antibodies (nanobodies)
- Select human monoclonal antibodies from patient-based libraries
- Improve properties of monoclonal antibodies
Recombinant antibodies are used as research reagents, reference reagents and antibody standards for diverse applications. Recombinant antibody technology can often generate more robust reference reagents that can be more precisely quality controlled and is particularly useful for areas historically dependent on difficult to source materials from human donors or from unstable hybridomas. We are also increasingly using
next generation sequencing to assist with recombinant antibody discovery.
We can produce recombinant antibodies, engineered antibody and protein variants at small scale, using microbial systems (batch and fermentation) and mammalian expression systems (transient and stable production in a bioreactor). We purify these antibodies using various techniques, predominantly affinity, ion exchange and size exclusion chromatography.
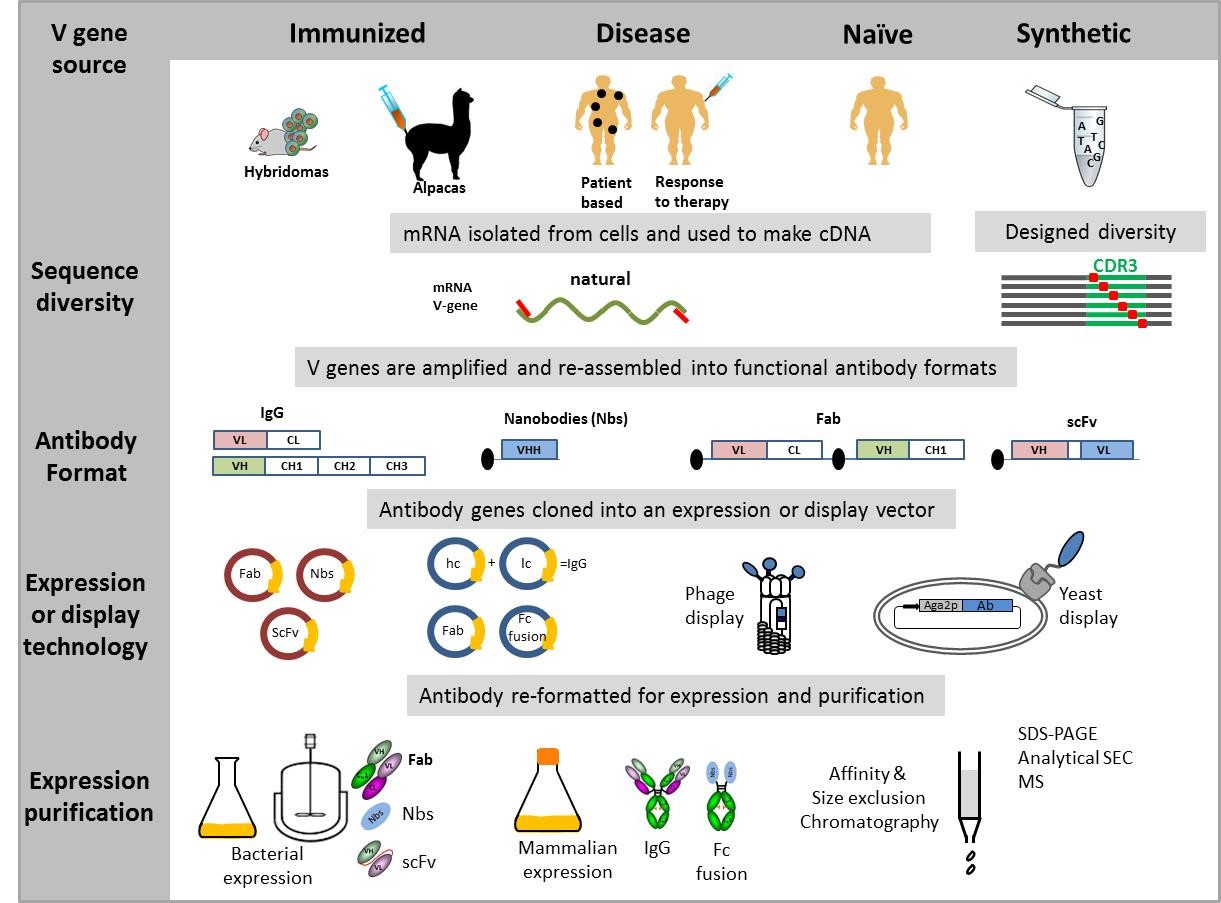
Some examples of active areas of research:
- cross-reactive nanobodies for influenza vaccine potency testing
- patient derived human monoclonal antibodies to study immunogenicity to therapeutic monoclonal antibodies
- isotype variants of therapeutic monoclonal antibodies
- conversion of critical hybridoma reagents into recombinant antibodies for use in regulatory potency assays
- cell lines expressing markers/receptors for bioassay development
Epitope mapping
The identification of epitopes is critical for understanding protein structure and function. We have established expertise in epitope mapping and antigenic fingerprinting of both monoclonal antibodies and polyclonal immune sera. We combine biomolecular display technologies (Phage display and yeast display) and next generation sequencing to map both linear epitopes and conformational epitopes. For example working closely with the Influenza Research group, we have used these methods to map the epitopes of nanobodies to influenza HA which are being explored for use in new vaccine potency assays (Gaiotto and Hufton, 2016).

Control
We perform post marketing surveillance on licensed therapeutic mAbs through European centrally authorised products (CAP) testing.The commercial success of therapeutic monoclonal antibodies has meant that there is an increase in the number of reported cases of fraudulent activity. As part of the MHRA we also support counterfeit testing of seized mAbs including:
- Product-specific potency bioassays such as inhibition of proliferation, apoptosis, binding, ADCC and CDC assays
- Tests for protein content
- High performance liquid chromatography (HPLC)