Therapeutic immunoglobulins and allergens
Therapeutic immunoglobulins are highly purified products made from pooled plasma collected from thousands of human donors. They are produced for either intramuscular or intravenous administration and are used for a range of therapeutic effects. Intramuscular immunoglobulins can be used to treat conditions such as hepatitis B, rabies, tetanus varicella zoster and anti-D. Intravenous immunoglobulins (IVIG) are used to treat primary immunodeficiencies and idiopathic thrombocytopenia (ITP) and are increasingly used for secondary immune deficiencies, haematological diseases and autoimmune diseases.
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Most humans mount significant Immunoglobulin E responses only as a defence against parasitic infections. However, some individuals may respond to many common environmental antigens. In these individuals, non-parasitic antigens stimulate inappropriate IgE production, leading to type I hypersensitivity. Diagnosis of allergic patients is carried out in specialist clinics using skin-prick tests with small amounts of allergen. De-sensitisation treatment involves a program of administering increasing doses of the allergen to an individual to stimulate a more cellular immune response. NIBSC provides a range of allergen standards that are used to calibrate the allergens used in the diagnostic and immunotherapy treatments. Some of the products marketed in the UK are also batch released at NIBSC.
What we do
Control
NIBSC serves as the UK’s Official Medicines Control Laboratory (OMCL) for biological medicines. Find out more about our control testing.
National Control Laboratory testing
Immunoglobulins can become damaged or denatured, due to exposure to extreme conditions such as pH and temperature or contaminating enzymes, or contaminated with agents such as viruses from the plasma donors. This can affect product quality.
European Pharmacopoeia (EP) monographs help control for unwanted characteristics in therapeutic immunoglobulins such as fragmentation, aggregation, agglutination activity, thrombogenic activity and viral contamination, requiring a range of tests to be carried out on every batch before release.
We test all therapeutic immunoglobulins for:
- protein content
- protein composition – by SDS poly-acrylamide gel electrophoresis (SDS-PAGE)
- distribution of molecular size – by size-exclusion high pressure liquid chromatography (SE-HPLC)
- appearance
- solubility – only on freeze-dried preparations
- potency – only for specific immunoglobulins such as hepatitis A or B, anti-D, tetanus, rabies or varicella zoster
- anti-D antibodies in human immunoglobulins for intravenous administration –except in anti-D immunoglobulin preparations
We also test for viral markers, hepatitis B surface antigen (HBsAg), Human Immunodeficiency virus (HIV) antibody and hepatitis C Virus RNA, in plasma pool samples used to make therapeutic immunoglobulins.
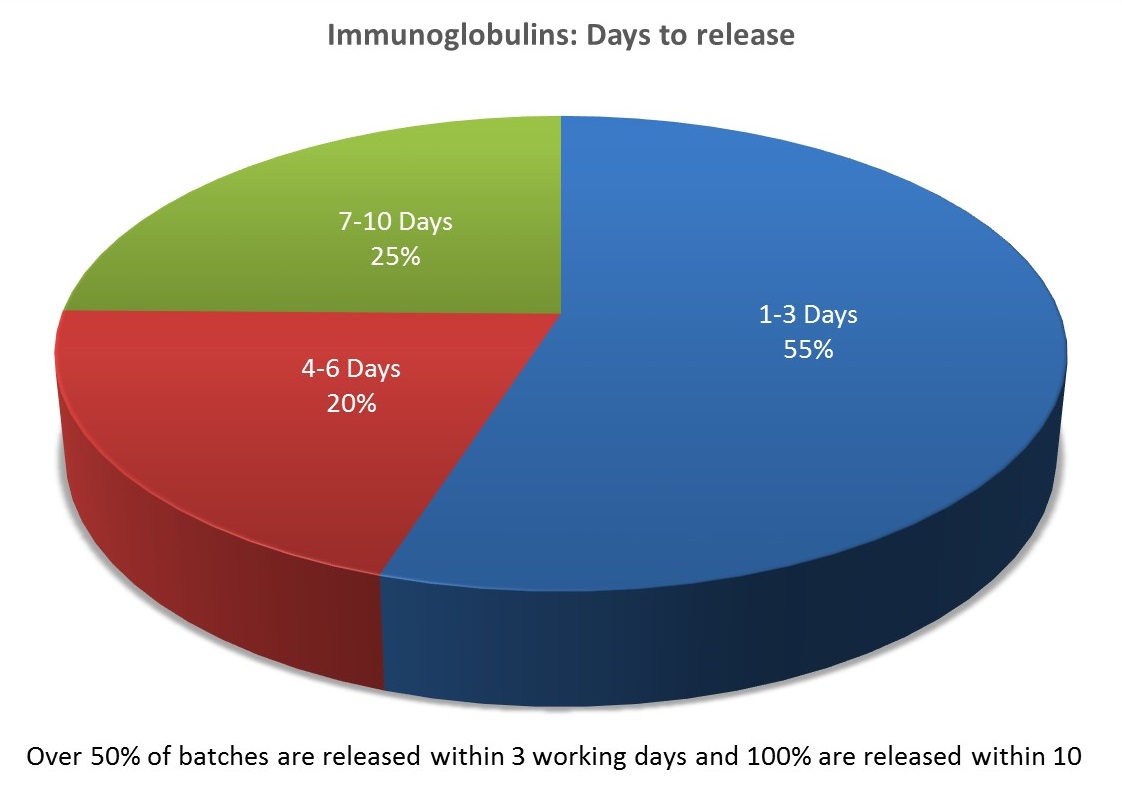
We aim to release all batches submitted for independent batch certification within ten working days of receipt.
Allergen products such as bee and wasp venom extracts as well as grass pollen extracts are also batch released by this section.
Adverse events
National Control Laboratory testing helps to ensure safety and efficacy of biological medicinal products, but adverse reactions can still happen. In these cases, NIBSC works with manufacturers and key stakeholders to understand the issues and provide solutions in the form of better assays, standards and other reagents.
We’ve been involved in investigating several incidents relating to therapeutic immunoglobulins such as:
- in 2009 when adverse events associated with an Anti-D product used for ITP patients – there were some deaths and the licence was withdrawn for the product across Europe
- in 2010-13 when thromboembolic adverse events seen with an IVIG product – 9 cases associated with 7 batches in USA – the FDA recalled the 7 batches and 24 others. France, Germany, Sweden and UK withdrew 80 batches pending an investigation and we led the way with a new standard to validate a new OCABR test
- in 2013 when increasing numbers of adverse events seen with several IVIG products relating to haemolytic events and a corresponding increase in anti-A and anti-B titres observed in new generation IVIG products around the world – NIBSC introduced the anti-A and anti-B direct agglutination assay as a mandatory independent batch release test from January 2014
Standardisation
We produce a range of biological standards and reference preparations for therapeutic immunoglobulins and allergens. We are currently working on standards for the analytical method, size-exclusion high performance liquid chromatography (SE-HPLC).
Our reference materials
Immunoglobulins and Immune sera
Allergens
Anti-allergens
Research
Recent research activities involve the use of analytical methods related to our control testing work. We’re working with colleagues on a standardisation project on the biosimilar monoclonal antibodies for Infliximab. Find out more about this project.