Haemophilia A and Factor VIII
Blood coagulation is a rapid series of complex reactions, which triggers “haemostasis”, a process by which the body responds to prevent blood loss. This occurs by a blood clot forming in a blood vessel at the site of an injury.
A series of proteolytic reactions takes place with a stepwise activation of coagulation factors – the ’coagulation cascade’ – which ultimately results in a fibrin clot forming.
Coagulation disorders can lead to excessive bleeding – this happens with haemophilia A – or excessive clotting such as in deep vein thrombosis.
Haemophilia A is a recessive X-linked bleeding disorder caused by a deficiency or a functional abnormality of coagulation co-factor Factor VIII (FVIII).
It affects 1 in 5000 males resulting in bleeding in joints, muscles and soft tissues. It is usually treated by prophylactic infusions of FVIII therapeutic products.
What we do
Our laboratory focuses on the diagnosis and treatment of bleeding coagulation disorders, in particular haemophilia A and diseases relating to clot formation.
We carry out the Institute’s core functions of standardisation and therapeutic product control testing. Both are underpinned by related research and developmental activities.
Standardisation
The complex structure and function of coagulation factors – coupled with their low concentrations in blood plasma – makes it virtually impossible to quantify them by physico-chemical means.
The estimation of coagulation factors therefore relies on the principle of comparative bioassay relative to a reference standard containing a known amount of analyte. Reference standards prepared locally can provide consistency and continuity of testing within a single laboratory but do not address the issue of harmonisation in testing between multiple laboratories.
International Standards (IS) established by the World Health Organization (WHO) fulfil this role by providing a common single route of calibration for all local and secondary working reference standards.
The first IS for a coagulation factor – factor VIII (FVIII) – was established in 1971 in response to the need for harmonisation in the potency labelling of the ‘new’ therapeutic concentrates. This approach has subsequently been applied to most plasma coagulation factors
Factor VIII
Our laboratory’s primary responsibility is for standardisation of FVIII – both plasma-derived and recombinant – in therapeutic concentrates, in particular for the development of new and upkeep of current WHO IS and British Standards for FVIII Concentrate.
The current WHO 8th IS (07/350) for FVIII concentrate is the only one in a series of ISs for which there is a complete agreement in FVIII potency between one-stage clotting and chromogenic methods.
However, as new modified/longer-acting FVIII products with extended half-life (EHL) emerge, there have been assay discrepancies and standardisation issues which will need to be addressed.
Field studies of FVIII concentrates
We have also carried out a series of field studies on behalf of the FVIII/FIX subcommittee of the International Society for Thrombosis and Haemostasis (ISTH) Scientific and Standardisation Committee (SSC) to assess any standardisation issues relating to assays on licenced FVIII products.
These studies perhaps give a truer picture about how assays perform routinely in laboratories where local methodologies, standards and calculation of results are used to estimate potencies in FVIII concentrates. It is envisaged that future such field studies would help to identify, and perhaps resolve, assay discrepancies and standardisation issues, particularly with the newer modified EHL FVIII therapeutic products.
Factor XIII and Fibrinogen
Contract testing
We undertake contract testing using a variety of validated assays in the Haemophilia areas as well as Gene and Cell therapy areas.
Contract manufacture of reference standards
We undertake contract manufacture and testing of product specific reference standards, in order to help customers resolve any assay discrepancy issues, particularly in the Haemophilia area.
Control testing
Factor VIII concentrates
As the UK Official Medicines Control Laboratory (OMCL), National Control Batch Release testing of FVIII therapeutic concentrates is carried out as part of the Institute’s statutory function. In addition to product testing and release for Great Britain, we also carry out testing for non-EU markets on behalf of manufacturers.
The products tested consist of freeze-dried, plasma-derived FVIII purified from large pools of plasma from donors. The plasma-derived FVIII concentrates will have undergone viral inactivation and are used primarily for replacement therapy in treating haemophilia A patients.
They are also used in immune tolerance induction (ITI) regimens to treat patients that develop antibodies or ‘inhibitors’ – alloantibodies or autoantibodies – to the FVIII molecule.
Control Tests performed
- FVIII chromogenic assays
- VWF: antigen assays
- VWF Ristocetin co-factor assay
- solubility and appearance tests
- protocol review
Research and development
Haemophilia A and Factor VIII Immunogenicity
Haemophilia A is a recessive X-linked bleeding disorder caused by a deficiency or a functional abnormality of coagulation co-factor FVIII.
It affects 1 in 5000 males resulting in bleeding in joints, muscles and soft tissues. It is usually treated by prophylactic infusions of FVIII therapeutic products.
However, a major complication of such substitutive treatment is the development of an immune response – developing neutralising antibodies – towards the FVIII molecule, with most of the alloantibodies developed being directed to the functional epitopes (A1, A3 and C2 domains) of the FVIII molecule.
Antibodies inhibiting FVIII activity – ‘inhibitors’ – preclude further use of FVIII, leaving patients in a life-threatening situation. About 30% of severe haemophiliacs have these antibodies.
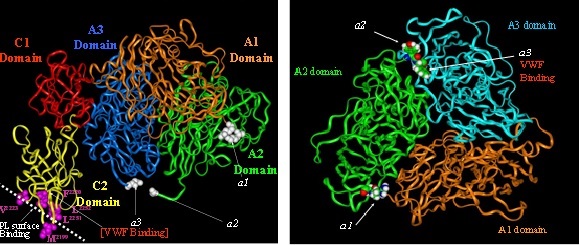
Molecular model and domains of FVIII molecule
FVIII autoantibodies can also develop in non-haemophiliacs, in a variety of clinical settings such as the postpartum period and chronic lymphocytic leukaemia.
Using recombinant FVIII has not reduced the incidence of inhibitors.
Furthermore, the methods used to prepare FVIII or reduce viral transmission carry the risk of altering the immunogenicity of the molecule.
Present treatments to eradicate FVIII inhibitors are unsatisfactory. They rely on methods such as infusion of large doses of FVIII – alone or in combination with non-specific immunosuppressive agents.
The cost of these treatments is prohibitively high and they tend to be effective only in patients with recent, low-titre, inhibitors.
Inhibitor development in up to 20% of patients is related to the gene defect – intron 22 inversion of the FVIII gene. Preventing inhibitors from developing is currently not feasible, as criteria to identify patients at risk are not yet fully defined.
There is therefore, a need for alternative, more efficient therapies which would be both specific and cost-effective. But before this can happen, we need to understand precisely the mechanisms of inhibitor development and by which FVIII interacts with other analytes in blood.
In addition, current methods to measure FVIII inhibitors are unsatisfactory and developing new assays which can measure inhibitors accurately and reproducibly is critical for any meaningful data for diagnosis and therapy.
Other areas of research
We are working on a number of different research areas to address the above FVIII immunogenicity problems:
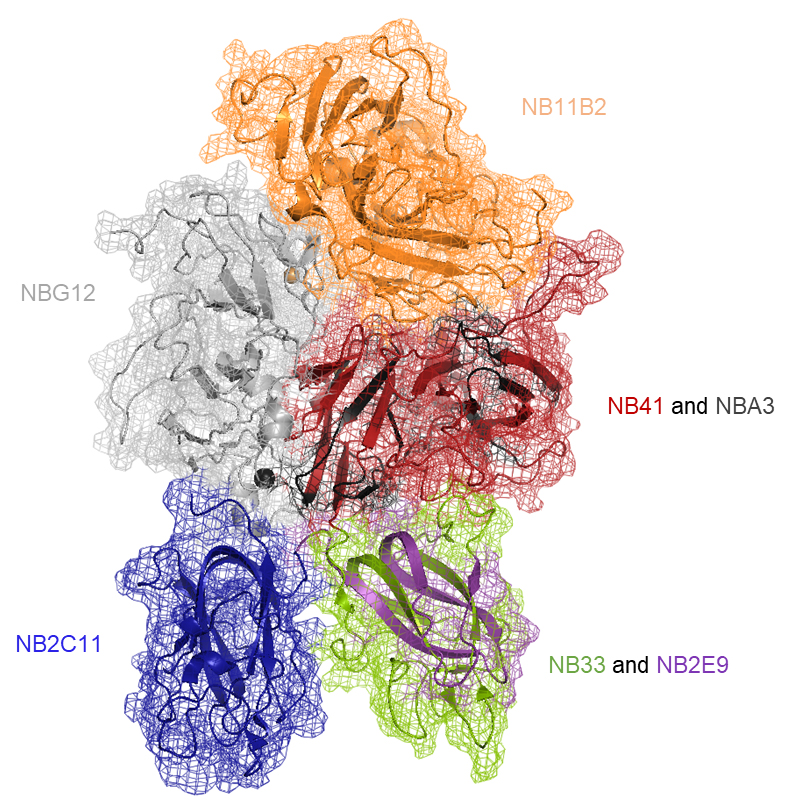
- development and characterisation of neutralising recombinant monoclonal antibodies to FVIII for standardisation of inhibitor assays. A panel of 7 rIgG4s (NB2C11, NB11B2, NB33, NB41, NB2E9, NBA3 & NBG12) have been produced in our laboratory covering the A2, A3, C1, & C2 domains of the FVIII molecule. These antibodies are also useful for basic research purposes.
Schematic of the crystal structure of FVIII
showing domains targeted by each rIgG
- assessment of the effects of manufacture-in-process structural and allosteric modification (e.g. glycosylation) of recombinant FVIII products on its functional activity and immunogenicity.