Fibrinolysis
Cardiovascular disease and thrombolytic drugs
Cardiovascular disease – including heart disease and stroke – continues to be the leading cause of death worldwide. The World Health Organisation (WHO) says it accounts for 23.6% of total deaths and 37.7% of deaths in Europe. The financial cost of cardiovascular disease is projected to more than double by 2030.
In most cases, acute cardiovascular events arise from the development of an unwanted thrombus formed by rupture of an atherosclerotic plaque. Thrombolytic drugs or ‘clot busters’ are biological agents used to break down unwanted clots.
Our group
The work of the fibrinolysis group includes maintaining a range of WHO International Standards in the field of thrombolytic drugs. We work with several global collaborators to study fundamental questions around the regulation of enzymes used as plasminogen activators that promote fibrinolysis.
Common thrombolytic agents we deal with include:
- tissue plasminogen activator
- streptokinase
- urokinase plasmingen activator
- plasmin
- alpha-1-proeinase inhibitor
- C1-inhibitor
Research
Fibrinolysis Assays
The lab has a longstanding interest in optimising fibrinolysis assays [1, 2], used to measure the activity of thrombolytic drugs and for the development of WHO International Standards.
More recently we have studied the standardisation of data analysis from haemostasis assays and developed a number of online Shiny apps [3, 4]. These apps can be used with various haemostasis assays and they have been applied in published standardisation and research projects [5-8].
A summary of apps can be found here at https://drclongstaff.github.io/shiny-clots/
Clot lysis curve analysis using ClotlysisCL_2019

Neutrophil Extracellular Traps (NETs) and Fibrinogen
NETs are mixtures of DNA, histones and enzymes expelled by dying neutrophils as part of an ancient immune defence mechanism to fight invading pathogens. In vitro NETosis can be triggered by PMA or calcium ionophores, like ionomycin. Histones are highly charged cytotoxic proteins and we find they also trigger NETosis [9]. There is a risk of positive feedback where NET formation and histone release could cause widespread collateral damage. However, fibrinogen reduces NET generation and histone cytotoxicity [9].
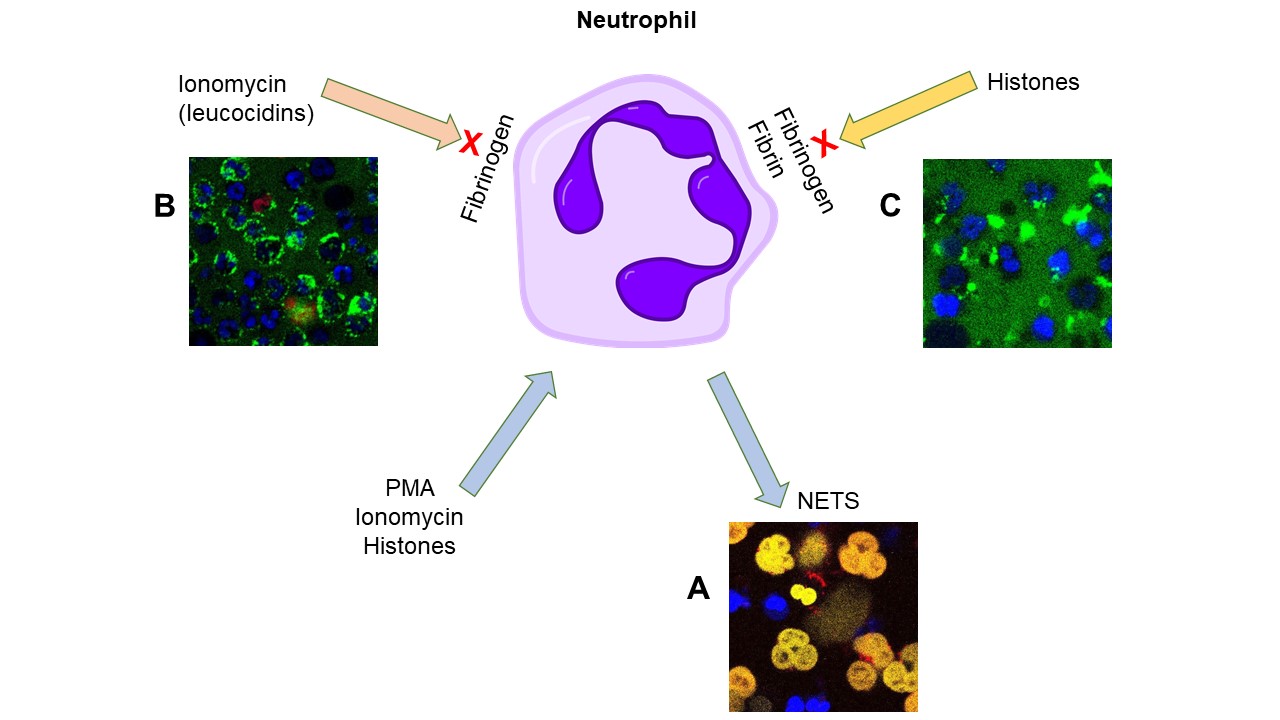
A NETosis can be triggered by PMA, ionomycin or histones (here histones are red, NETs stained yellow and intracellular DNA is blue)
B After ionomycin treatment, neutrophils bind fibrinogen (labelled green) via Mac-1 cell surface receptors and NETosis is delayed.
C Histones are prevented from binding to and forming pores in neutrophil membranes by fibrinogen or fibrin. Complexes of histones-fibrin(ogen) (shown in green) do not trigger NETosis.
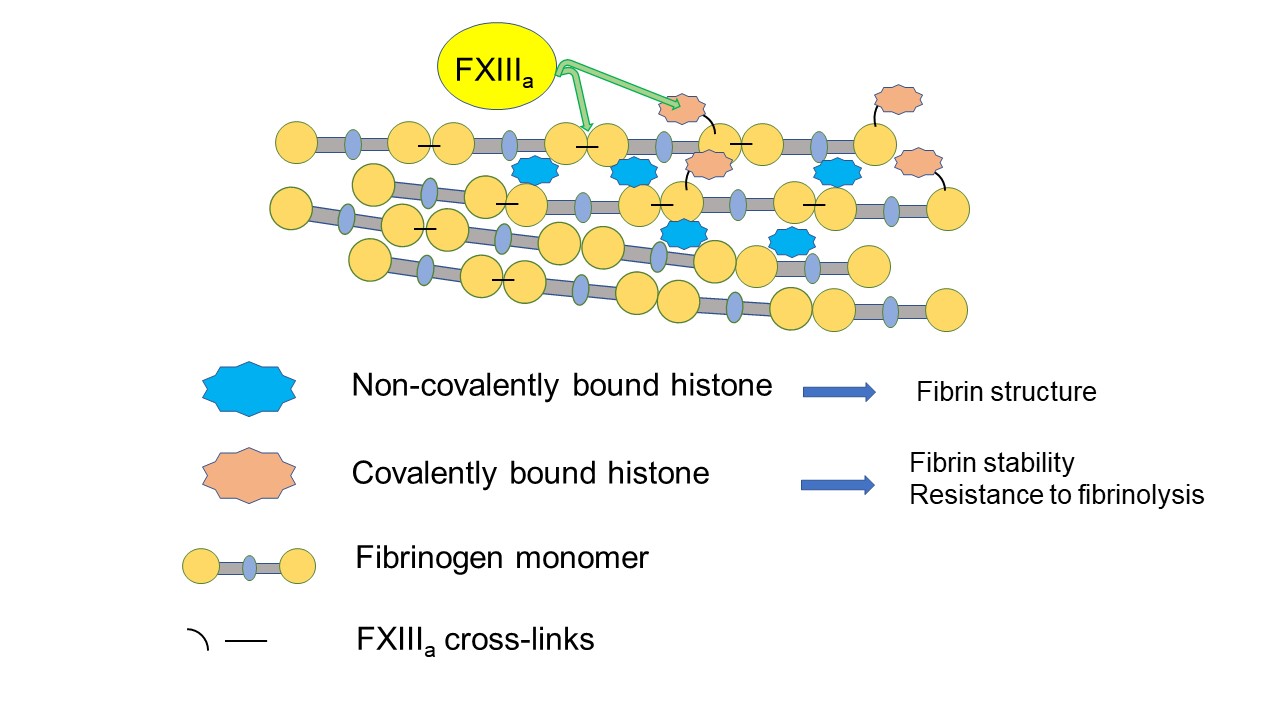
Histones are also known to interact with fibrin and affect structure and properties [10]. Recently we have found that histones can be cross-linked into fibrin by FXIIIa [11]. This covalently bound histone affects fibrin stability and fibrinolysis in a different way to histones that are associated with fibrin but not cross-linked [11].
References
1. Longstaff, C., Measuring Fibrinolysis. Hamostaseologie, 2021. 41(1): p. 69-75.
2. Longstaff, C., Measuring fibrinolysis: from research to routine diagnostic assays. J Thromb Haemost, 2018. 16(4): p. 652-662.
3. Longstaff, C. shiny-clots. 2021 Available from: https://drclongstaff.github.io/shiny-clots/.
4. Longstaff, C. Development of Shiny app tools to simplify and standardize the analysis of hemostasis assay data: communication from the SSC of the ISTH. J Thromb Haemost, 2017. 15(5): p. 1044-1046.
5. Locke, M., C. Longstaff, and P. Rigsby, An international collaborative study to establish the WHO 3rd International Standard for Thrombin: Communication from the ISTH SSC subcommittee on factor XIII and fibrinogen. J Thromb Haemost, 2021. 19(3): p. 852-858.
6. Kearney, K.J., et al., Kallikrein directly interacts with and activates Factor IX, resulting in thrombin generation and fibrin formation independent of Factor XI. Proc Natl Acad Sci U S A, 2021. 118(3).
7. Roberts, G., B. Fox, and C. Longstaff, Development of an updated assay for prekallikrein activator in albumin and immunoglobulin therapeutics. Vox Sang, 2020.
8. Locke, M., et al., An international collaborative study to establish the WHO 4th International Standard for Streptokinase: Communication from the SSC of the ISTH. J Thromb Haemost, 2020. 18(6): p. 1501-1505.
9. Locke, M., et al., Fibrinogen protects neutrophils from the cytotoxic effects of histones and delays neutrophil extracellular trap formation induced by ionomycin. Sci Rep, 2020. 10(1): p. 11694.
10. Longstaff, C., et al., Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J Biol Chem, 2013. 288(10): p. 6946-56.
11. Locke, M. and C. Longstaff, Extracellular Histones Inhibit Fibrinolysis through Noncovalent and Covalent Interactions with Fibrin. Thromb Haemost, 2020. 121(4): p. 464.