Bleeding disorders and procoagulant therapeutics
The bleeding disorders and procoagulant therapeutics group is responsible for maintaining a portfolio of WHO International Standards for potency assignment to therapeutic concentrates and plasma standards used in the treatment and diagnosis of haemostasis disorders. The group is accredited to EN ISO 17025 to carry out National batch release on Virus Inactivated (VI) Human Plasma, and performs research to support these standardisation and control activities. We also have research interests in external factors, such as bacterial virulence proteins, that modulate physiological haemostasis processes and cause disease.
What we do
Our work covers:
Standardisation
We produce standards to support bleeding disorder diagnosis and therapy. See our full list of haemostasis standards
von Willebrand disease and von Willebrand Factor
The diagnosis of von Willebrand disease (VWD), one of the most common bleeding disorders, relies on the measurement of von Willebrand factor (VWF) in blood plasma.
Our WHO 6th IS Factor VIII / von Willebrand factor, plasma (07/316) supports global harmonisation of diagnosis by defining the International Unit (IU) for:
- von Willebrand factor function as Ristocetin cofactor and collagen binding
- von Willebrand factor antigen
- von Willebrand factor propeptide - used to identify VWD sub-types and as a marker of endothelial perturbation
von Willebrand factor (VWF) concentrate
Replacement therapy for the bleeding disorder, von Willebrand disease (VWD) is undertaken using purified concentrates of VWF, some of which also contain coagulation factor VIII. The WHO 2nd IS VWF Concentrate (09/182) is used for the potency labelling and characterisation of VWF in these products by defining the IU for:
- VWF: ristocetin cofactor
- VWF: collagen binding
- VWF: antigen
Factors VII and VIIa
Factor VII concentrate
The WHO 2nd IS for factor VII concentrate (10/252) is used for potency assignment to human coagulation FVII concentrate preparations used to treat FVII deficiency, and for FVII-containing prothrombin complexes used for the reversal of anticoagulant treatment.
The 2nd IS was calibrated in an international collaborative study using clotting and chromogenic assay methods. Two candidates (A – 10/250 and B – 10/252) were included in the study and statistical analysis of the results revealed potencies determined by clotting and chromogenic methods were significantly different for both candidates.
Candidate A (10/250)

Candidate B (10/252)
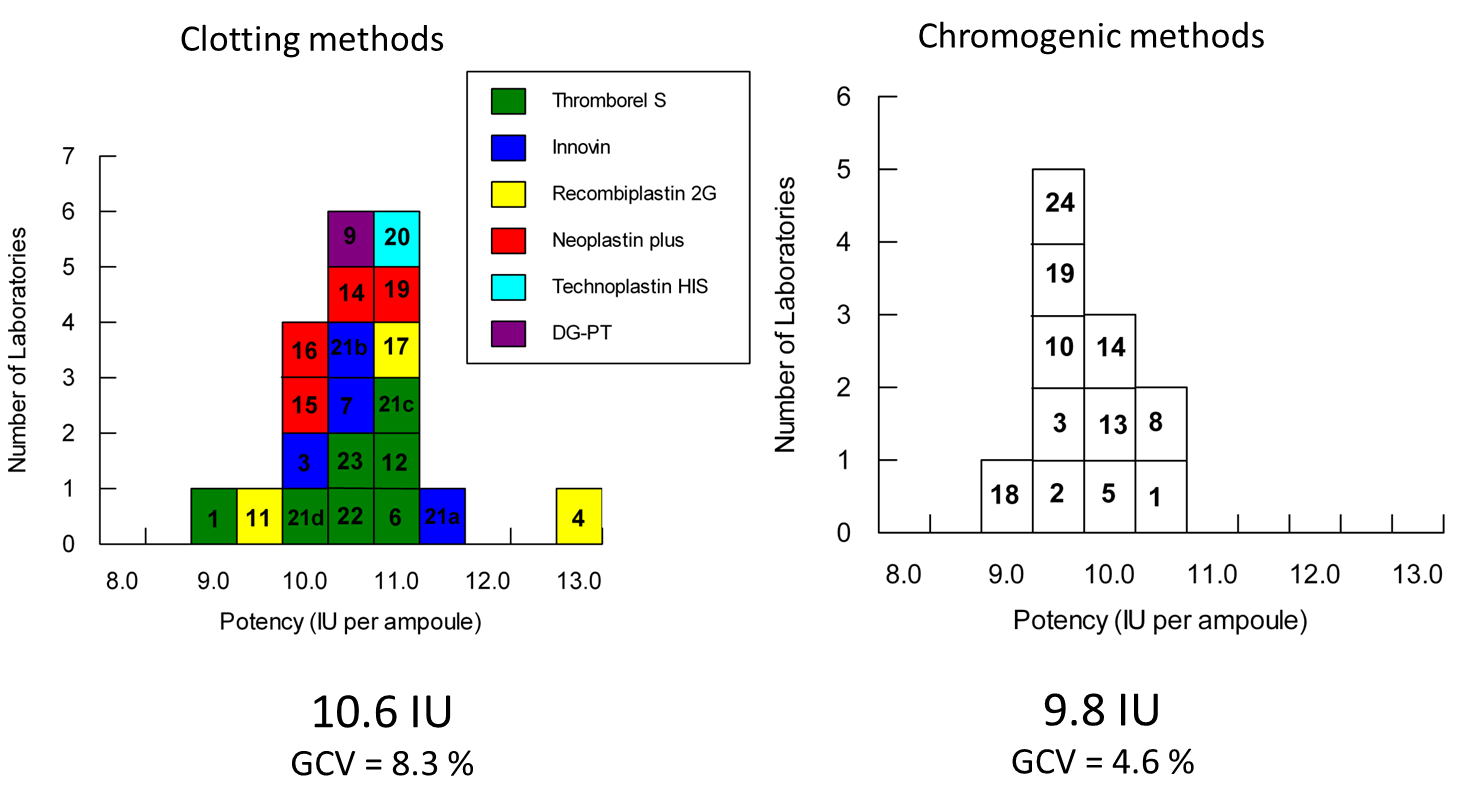
In addition there was a significant assay method bias within the clotting results for candidate A, with laboratories using recombinant thromboplastin reagent producing lower potencies compared to those using a natural purified thromboplastin.
Because of the assay method bias observed with Candidate A and recombinant thromboplastin reagents, and the larger discrepancy between clotting and chromogenic methods for Candidate A, candidate B (10/252) was established as the WHO 2nd IS for factor FVII concentrate. The difference between the potency estimates by the different methods was however too large to reconcile. The 2nd IS (10/252) was therefore established with different potency values for chromogenic and clotting methods.
The discrepancies identified in this study between the candidate materials, and between the different assay methods and reagents used, could have a significant impact on potency assignment to different therapeutic products. An investigation is underway to identify the important factors that influence potency assignment to FVII concentrate products.
Activated factor VII (FVIIa) concentrate
Inhibitory antibodies to FVIII develop in around 30% of Haemophilia A sufferers as a result of exposure to infusions of FVIII concentrates:
- these ‘inhibitors’ reduce the efficacy of FVIII replacement therapy
- activated factor VIIa can bypass the requirement for FVIII and control bleeding episodes in these patients by directly activating factor X
The WHO 2nd IS Factor VIIa Concentrate (07/228)defines the IU for clotting activity in FVIIa therapeutic concentrates. At present a recombinant FVIIa product is predominately used however there are a number of new and modified products in development by manufacturers. Assay and reagent discrepancies between the WHO IS and any individual or class of new product that is not identified could result in a potency shift that affects labelling and dosing of therapeutic FVIIa products.
An NIBSC study is planned, with the co-operation of the product manufacturers, to investigate the relationship between the WHO IS and new/modified products in a range of different assay systems. This undertaking will be essential for understanding and regulating the use of the WHO IS as new therapeutic products emerge.
Factor Xa
Blood coagulation factor Xa (FXa) plays a critical role in the coagulation cascade, holding a central position that links the extrinsic and intrinsic pathways. Measurement of FXa is a regulatory requirement for FEIBA (Factor Eight Inhibitor Bypassing Activity), an activated prothrombin complex concentrate (APCC) used in the treatment of haemophilia patients with inhibitory antibodies against FVIII.
A WHO Reference Reagent for activated blood coagulation factor Xa (FXa), human, (15/102) was established in 2017 calibrated against, and as a replacement for, a non-WHO reference material for FXa (75/595) of bovine origin.
Although measurement of FXa in FEIBA is at present the primary regulatory use for the WHO FXa reference reagent, potential future uses include standardising the measurement of FXa as a contaminant in non-activated products such as prothrombin complex concentrates (PCC), used in the reversal of anticoagulant therapy. Standardising the biological activity of direct FXa inhibitors, used in anticoagulation therapy, is another significant potential use which would require a human FXa standard due to potential differences in the active site inhibition between bovine and human FXa.
Establishing a WHO RR for FXa will provide an opportunity to investigate the development of a WHO International Standard (IS). To achieve this, the utility of the RR for potency estimation of human FXa must be assessed in the full range of assay methods in contemporary use. Feedback from new users of the FXa RR is requested for this purpose, including on any issues arising with any particular assay system. This information may then inform the nature of a future collaborative study to establish a WHO IS for FXa, Human.
Research
Bacterial virulence factors and haemostasis
Many bacteria express virulence factors that hijack the mammalian haemostasis system such as proteins that activate human plasminogen to degrade fibrin.

Much of our understanding of the mechanism of plasminogen activation by streptokinase (SK) is based on a human isolate of the Group C streptococcus S. equisimilis (H46A). This Group C SK has been exploited for decades as a thrombolytic drug used in the treatment of myocardial infarction
Staphylococcus aureus produces proteins that use the host haemostasis system to both create fibrin (staphylocoagulase and von Willebrand Factor binding protein (vWbp)) and to destroy fibrin (staphylokinase):

Staphylocoagulase (SC) binds to and activates prothrombin (ProT) through a non-proteolytic process called “molecular sexuality”. The SC-ProT complex converts fibrinogen to fibrin, circumventing the physiological coagulation cascade.
S. aureus is a frequent cause of human wound infections and impaired wound healing and is frequently seen in S. aureus-infected chronic wounds. Alterations in fibrin structure and function have implications for wound healing and may contribute to the establishment of S. aureus infections and diseases.
Our investigations have identified marked differences in the enzyme kinetics of SC-ProT, compared to thrombin, that result in different structural and physical properties of the fibrin product. Fibrin structure is known to affect biological function and the different structural and physical properties of the SC-ProT fibrin product could play an important role in the severity of S. aureus infections.
Turbidimetric clotting curves and fibrin product SEM for SC-ProT and ThrISTH/SSC
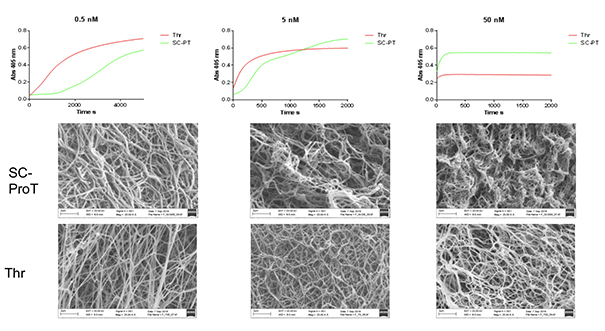
There are marked differences in the kinetics of fibrin polymerization by Thrombin and SC-ProT (staphylocoagulase-prothrombin complex) resulting in different structural and physical properties of the fibrin product.
SC-ProT fibrin has thicker fibres and is more porous than Thrombin fibrin, with higher concentrations of both producing thinner fibres and less porous clots.
SC-ProT fibrin clots are softer, easier to deform and less resistant to mechanical forces than Thrombin clots.
Fibrin structure is known to affect biological function and the different structural and physical properties of the SC-ProT fibrin product could play an important role in the severity of S. aureus infections.
Where Thrombin fibrin forms a protective barrier to Infection and resists shear; SC-ProT fibrin may serve as a deformable shell to enhance microbial replication and dissemination.