Endocrinology
The relationship between the structure and biological activity of many endocrine medicines is not completely understood and can be difficult to predict due to their complex and heterogeneous nature. Comparative bioassays that include an external reference standard are needed to assess their potency.
The standardisation and control of endocrine therapeutics dates back to 1925, when the first International Standard for insulin was prepared by Sir Henry Dale. However, the recent growth in the types of endocrine therapies, including biosimilar medicines, sequence and structural variants, or modified versions of natural proteins, mean that their standardisation and control is now more important than ever.
What we do
Our group test and characterise biological medicines and work to develop the most appropriate bioassays and reference standards for the control and standardisation of endocrine therapeutics and calibration of diagnostic assays.
Our research activities currently include assay development and investigation of the therapeutic applications of induced pluripotent stem cells (iPSC).
We can also provide expert scientific advice to manufacturers and other organisations on assessing the potency of therapeutic endocrine products.
For us to provide WHO International Standards for the diagnostic and therapeutic communities we need manufacturers and other interested parties to donate materials. If you’re able to donate materials or interested in taking part in collaborative studies, please contact us at enquiries@nibsc.org.
Standardisation
Endocrine therapeutics
We are responsible for a large catalogue of reference standards for endocrine hormones and hormone-like substances.
These reference materials are critical for the control strategies for therapeutic products and we work with a range of international stakeholders, including the World Health Organisation (WHO), the British, European and US Pharmacopoeias and a range of national and regional regulatory organisations, to provide both traceability and consistency in evaluation of the quality and potency of endocrine therapeutics.
For example, we develop and manage the WHO International Standard for Erythropoietin (EPO), one of the biggest selling biological medicines which is used to treat anaemia caused by chronic kidney disease or cancer chemotherapy.
Diagnostic tests
Diagnosis of endocrine disorders relies on the provision of reference standards for the calibration of immunoassays. These standards help to ensure harmonisation of different assays so clinicians are provided with accurate information to assist diagnosis and patient management.
For example, we develop and manage WHO International Standards for Prostate-Specific Antigen (PSA), used to diagnose and manage prostate cancer.
System Suitability Testing
Pharmacopoeial monographs use a number of different High Performance Liquid Chromatography (HPLC) methods to assess product quality, through detection of impurities (oxidised, aggregated and degraded forms), which can affect the safety and efficacy of biological medicines.
These methods often require manipulation of a chemical reference substance (CRS) to generate impurities and demonstrate that the system is performing adequately (often termed system suitability), or interpretation of a chromatogram provided with the CRS to identify a particular impurity. These interventions are subject to poor reproducibility, inaccuracies and inconsistencies between laboratories, which could be avoided by the provision of bespoke reference materials.
For example, we developed an oxidised peptide standard for Interferon β1a, a therapeutic glycoprotein which is used to treat multiple sclerosis. This material is used for the oxidised forms test in the British Pharmacopoeia monograph for Interferon β1a Injection:
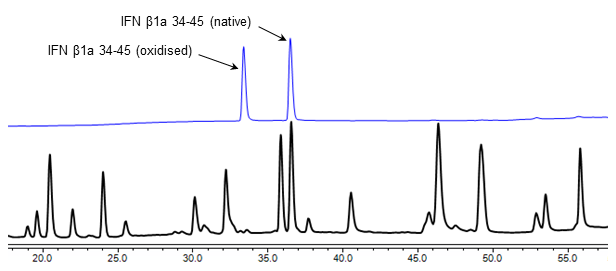
HPLC chromatograms of an Interferon β1a peptide map (black) alongside a proposed Interferon β1a oxidised peptide CRS (blue). The CRS is analysed alongside the peptide mapping sample, and facilitates identification of the native and oxidised forms of peptide 34-45. This enables the percentage of oxidised forms in Interferon β1a therapeutic preparations to be determined.
Control
We test counterfeit or seized endocrine products on behalf of the MHRA and carry out contract testing of endocrine and endocrine-related products for pharmaceutical and biotechnology companies.
Find out more about NIBSC control testing.
Research
Assay development
We are committed to developing new immunoassays, bioassays and physicochemical methods for the characterisation of novel reference standards and newly developed endocrine-related therapeutics and diagnostics.
Currently, cell-based in vitro bioassays are widely used in the biopharmaceutical industry to test potency, however these methods can be time-consuming and need improvement.
We’ve developed a way to use molecular biology techniques such as quantitative polymerase chain reaction (PCR) to measure bioactivity. Our work suggests this type of assay can be successfully used to detect and quantify antibodies against erythropoietin for diagnosis of pure red cell aplasia.
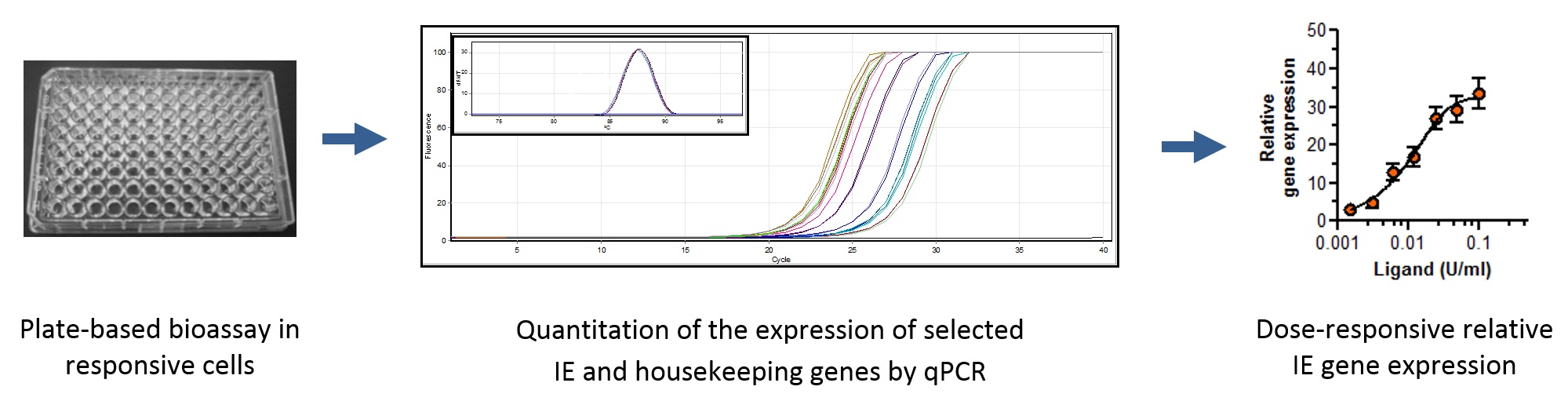
| Protein | Cell type | IE gene | Potential use | Reference |
|---|
| Interferon-β |
A549 |
IFI6 |
Alternative to bioassays measuring anti-viral activity |
Moore et al., 2008 |
| Thyroid-stimulating antibody |
FRTL-5
|
Nr4a1 (Nur77, NGFI-B) |
Detection of stimulatory/ blocking auto-antibodies |
Moore et al., 2014 |
| Erythropoietin |
UT-7/EPO |
EGR1 |
Detection of neutralising antibodies |
Ferguson et al., 2013 |
Quantification of immediate early (IE) gene expression to measure protein bioactivity using qPCR. This technique has been applied to several protein targets.
Stem cell research
With colleagues in the Division of Advanced Therapies we are part of a research programme investigating the therapeutic application of induced pluripotent stem cells (iPSCs).
As iPSC derivatives for cell therapies are likely to be used in an MHC matched transplant setting, they should provoke a limited immune response in the patient and therefore, avoid the need for immunosuppression upon transplantation. However, there is still some concern that, even with this approach, the specific cell derivative, or unexpected genetic or epigenetic changes arising during cell derivation, could make the cells immunogenic, and cause the patient’s immune system to respond upon transplantation.
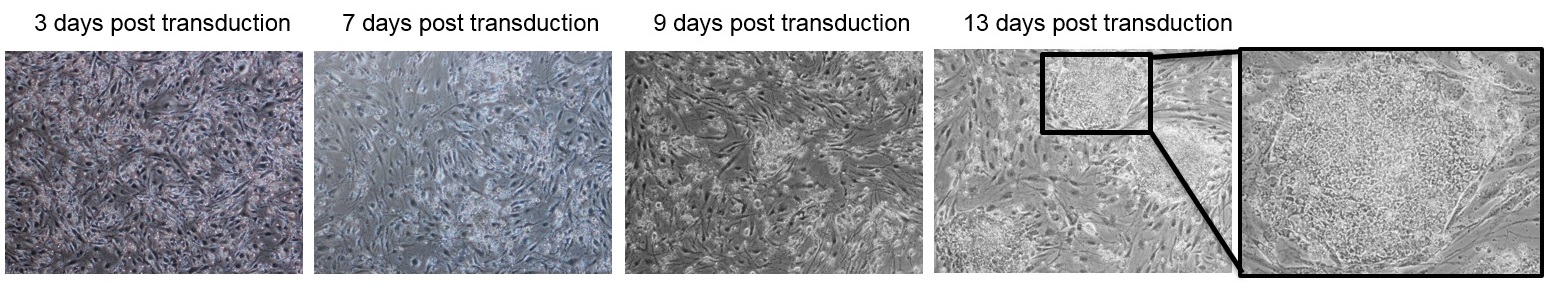
Generation of iPSCs from PBMC by reprogramming with Sendai virus vectors (CytoTune™, Invitrogen). Pictures show the development of stem cell colonies from PBMC transduced with the viral vectors encoding the reprogramming factors, Oct-4, Sox2, Klf4 and cMyc. Colonies at 13-15 days post-transduction are then selected for culture and the generation of an iPSC-line.
We are currently investigating the immunogenicity of different human iPSC derivatives and the use of therapeutic approaches, such as mesemchymal stromal cells, regulatory T-cells and co-stimulatory blockade, which could be used to reduce immune responses to iPS cell populations.