Flow Cytometry Reference Materials
We develop flow cytometry reference materials to standardise clinical diagnostic assays and harmonise flow cytometric analysis between laboratories. Existing flow cytometry reference materials cover the following areas:
Cross-matching reference materials
Flow cytometry cross-matching (FCXM) is routinely performed to match donors and recipients of transplants and in transfusion medicine. It determines the level of reactive antibodies in patients’ serum directed against donor HLA antigens – usually by using donor lymphocytes as targets. Presence of antibodies indicates that the recipient has been sensitised to donor antigens, increasing risk of rejection.
Transfusion-related acute lung injury (TRALI) can cause of illness and death following blood transfusion. It results from an immune reaction between the recipient and the transfused blood. Antibodies in donor plasma act against the recipient’s white cells and/or endothelial cells leading to leukocytes being trapped in the small blood vessels of the lung and damage to the pulmonary endothelium. This results in fluid and inflammatory cells leaking into the lungs. The antibodies are normally directed against the recipient’s human leucocyte antigen (HLA) class I, HLA class II or human neutrophil antigens (HNA).
Our flow cytometry cross-matching (FCXM) reference materials are developed as positive and negative run controls in assays for human leucocyte antigen (HLA) miss-match screening to minimise the risk of Transfusion Related Acute Lung Injury (TRALI).
View an overview of both available and in development FCXM reference materials.
Antibody and cell reference materials
We produce lyophilised antibodies and cell standards for enumeration of specific cell populations.
We also provide mouse anti-human CD4 monoclonal antibody conjugated to FITC, for use as a flow cytometry reference reagent for measurement of CD4 antigen on human leukocytes.
We have developed a stable reference material containing stabilised granulocyte colony-stimulating factor (G-CSF) mobilized apheresis cells that can be used as a quality control product for flow cytometry CD34+ stem cell counting. The CD34+ cell standard (available from USP catalogue) supports a US Pharmacopeia sub <1000> General Test Chapter for CD34+ cell enumeration by flow cytometry.
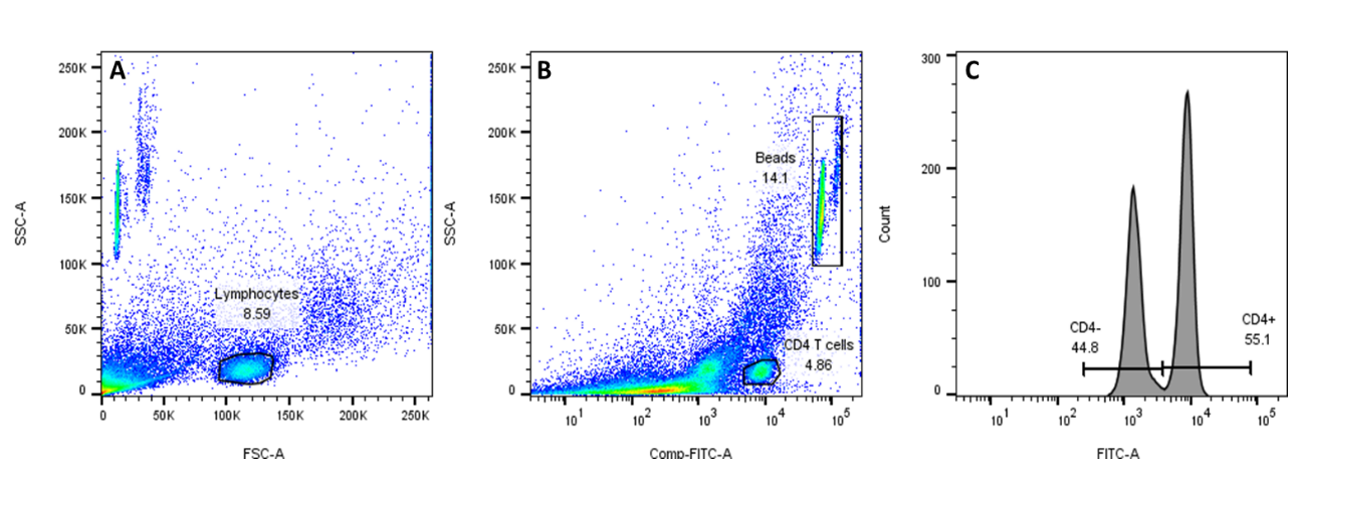
CD4 T cell counting using the FITC CD4 Positive Control Cells (SS-319/20) and TrucountTM beads
We have developed a cellular reference material for use as positive control for CD4+ and CD8+ T cell intracellular cytokine staining, a technique used to evaluate the efficacy and safety of novel candidate therapeutics at the pre-clinical stage and for immuno-monitoring during clinical trials.
These materials aim to address existing issues relating to variability in data from multi-centre clinical trials caused by difficulties in enumerating often low numbers of cytokine producing cells.