Standardisation science
Biological medicines, with a market worth nearly $200 billion in 2013, are a major player in the global pharmaceutical business.
Much of the material used is freeze-dried (lyophilised) to ensure adequate stability for storage and distribution, so understanding the freeze-drying process and its importance is critical for the regulation and control of biological medicines.
The Standardisation science group provides that knowledge in NIBSC, with a focus on preserving biological activity and delivering stable format biologicals.
Our work
We develop formulations and freeze-drying conditions to ensure that NIBSC can produce stable and suitable primary reference materials for the standardisation and control of biological medicines.
This is underpinned by activities to analyse and understand the freeze-dried state.
Our expertise and capabilities in understanding the molecular and physical properties of biological reference materials ensure that NIBSC has high assurance of success in delivering these key reference materials and those which we produce for collaborating organisations and companies seeking contract reference material manufacture.

Activities
In our core activity we deliver many trial freeze-drying studies each year across a very broad range of biological materials – such as purified proteins, antisera, complex mixtures, viruses, nucleic acids and complex carbohydrates and diverse formulations.
Understanding the impact of formulation on the key physicochemical properties is critical. We have analytical capabilities in these thermal analytical methods:
- modulated differential scanning calorimetry
- dynamic mechanical analysis
- differential thermal analysis
- impedance analysis
and also the orthogonal technique of freeze-drying microscopy which allows the lyophilisation process to be modelled in both temperature and vacuum conditions.
DoE study to optimize formulation of freeze dried lactate dehydrogenase
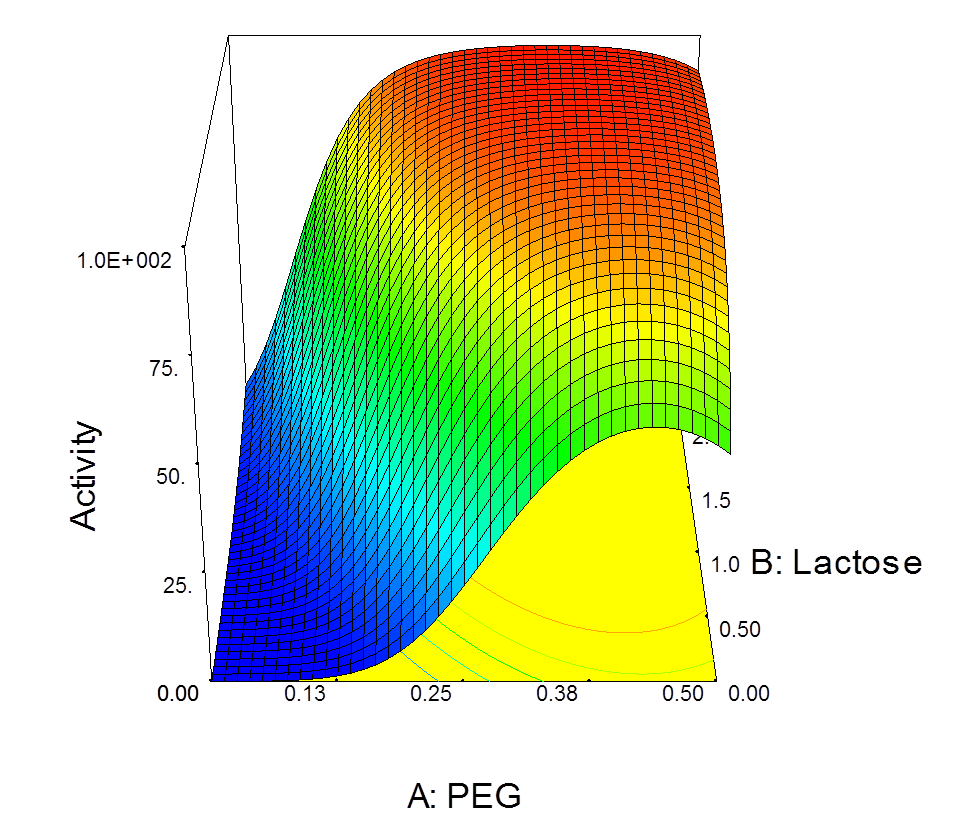
We have two pilot freeze-dryers which can deliver batches from a few dozen to up to 1500 ampoules or vials, with aseptic or environmentally-contained dispensing as an option.
Residual water is a key factor influencing the stability of lyophilised biologicals. We can determine the moisture content by:
- coulometric Karl Fischer titration
- vapouriser coulometric Karl Fischer titration
- non-invasive moisture determination by near infrared reflectance spectrometry (process sensors)
- automated coulometric Karl Fischer titration
- thermogravimetric analysis with evolved gas analyser
We can offer these services on a contractual basis, depending on commercial and Conflict of Interest limitations:
- thermal analysis of biological materials
- formulation development for biological materials
- freeze-drying cycle development
- stability analysis of freeze-dried formulations
Our research interests:
- formulation of biological and biopharmaceutical materials
- freeze-drying of novel/complex biological systems
- relationship between formulation and stability of liquid and freeze-dried materials
- use of Design of Experiment and Process Analytical Techniques in the freeze-drying process