Recombinant proteins
Our protein science laboratory maintains a facility for the preparation, purification and characterisation of recombinant proteins on a pilot scale.
This facility supports structure-function studies of biological medicines by allowing us to produce structural variants to analyse specific biological properties.
Key technologies include:
- soluble cytoplasmic bacterial expression
- cytoplasmic inclusion granule bacterial expression
- bacterial periplasmic expression
- transient and stable expression in Chinese hamster ovary (CHO) cells
- amplified antibody production in CHO cells
- batch and fermenter production
The various expression systems are supported by a range of preparation and purification methods including:
- chaotrope solubilisation and refolding of granule-expressed proteins
- affinity-tag purifications such as 6xHis
- semi-preparative scale size exclusion and high-performance ion exchange chromatography
- biological and physico-chemical analysis of purified proteins
Our current and recent projects include preparation of:
- recombinant anti-D IgM and site-directed structural mutants – CHO cell
- TGN 1412 IgG – sub-class variants and hinge-region mutants
- filgrastim – recombinant granulocyte colony stimulating factor (GCSF) and conformationally de-stabilised structural mutants of GCSF
Regulator requests for therapeutic proteins: the relationship between the conformation and biological activity of filgrastim
Higher order structure, including conformation, is considered a critical quality parameter of therapeutic proteins and is mandatory information in development of first use and bio-similar therapeutic protein drugs. The assumption is that the biological activity of a protein is directly dependent on its adoption of a ‘correct’ conformation.
Studies on the relationship between conformation and activity depend on the ability to induce conformational changes in proteins, and conventional approaches such as thermal or chemical denaturation are incompatible with bioactivity measurements.
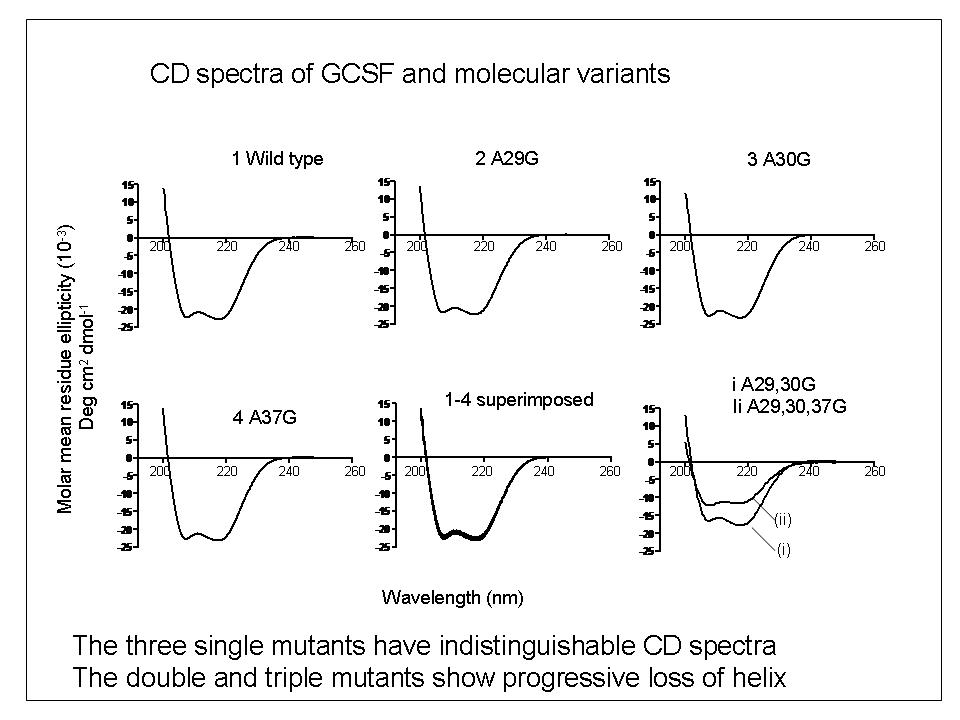
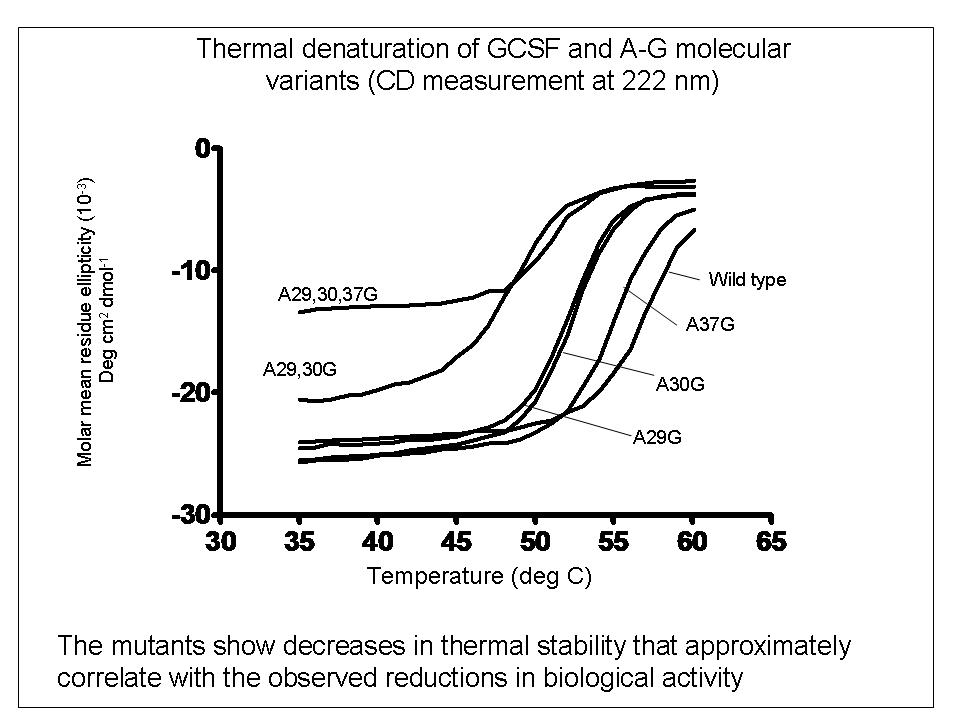
To explore the relationship between bio-activity and conformational studies, we studied variants of the therapeutic protein filgrastim (recombinant methionyl human granulocyte colony stimulating factor) which were mutated by the replacement of helical alanine residues with glycine to destabilise the conformation of the molecule.
In the GCSF A-G mutant series studied, single conformation-destabilising amino-acid substitutions significantly reduced the biological activity. These effects were not, however, correlated with changes in secondary structure measurable by far-UV Circular Dichroism (CD) spectroscopy.
We concluded that in this system, GCSF does not readily adopt a reduced-activity altered conformational state which can be detected by low-resolution techniques such as CD.
In contrast, reductions in biological activity do reflect reductions in conformational stability, possibly caused by time-dependent degradation of the protein in the cell-proliferation bioassay.
Although not a formal model of biosimilarity, we suggested that our results could inform the regulatory process in determining appropriate experimental approaches to meeting regulatory requirements for higher order structural analysis of therapeutic proteins.