Factor VIII Intron 22 inversion (F8)
Haemophilia A
Haemophilia A (OMIM #306700) is a hereditary bleeding disorder and occurs in about I in 5 000 to 10 000 male births.
The phenotypic manifestations are partial or complete absence of circulating factor VIII (FVIII) – a coagulation factor protein – leading to frequent spontaneous bleeding into the joints, muscles and internal organs.
Correct molecular diagnosis, especially of the female carriers, is of paramount importance. In addition to transmitting the defective gene, approximately 10% of the female carriers also have low circulating levels of FVIII and therefore are at risk of bleeding.
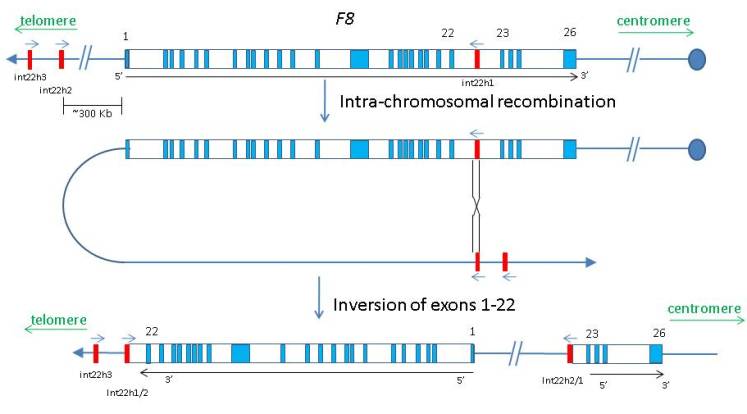
The intron 22 inversion mutation of the F8 gene accounts for 50% of severe haemophilia A, the most common X-linked congenital coagulation bleeding disorder. The inversion is caused by an intra-chromosomal recombination event between a 9.6 kb sequence within the intron 22 of the F8 gene and one of the two closely related inversely orientated sequences located about 300 kb distal to the F8 gene, resulting in an inversion of exons 1 to 22 with respect to exons 23 to 26.
Genetic analysis of the intron 22 inversion is challenging, involving technically demanding methods such as Southern blotting and long-distance PCR.
1st International Genetic Reference Panel for Factor VIII Intron 22 Genotyping
The panel comprises four human genomic DNA samples: normal male, normal female, carrier female, affected male. Samples are presented as approximately 20-30 μg freeze-dried genomic DNA in glass ampoules. The collaborative validation study data indicates that these materials are suitable for use in long-distance PCR and Southern blot tests.
How to order
To place an order please login to your account or sign up for a new account.
For scientific enquiries contact the team at grmteam@nibsc.org.